Compliance in patients with dietary hyperoxaluria: A cohort study and systematic review
Derek B. Hennessey *, Ned Kinner , Gilert Rice ,Dvid Curry , Siohn Woolsey , Brin Duggn ,c
a Department of Urology, Belfast City Hospital, Belfast, United Kingdom
b Department of Urology, Austin Hospital, Melbourne, Australia
c Department of Urology, Ulster Hospital, Belfast, United Kingdom
KEYWORDS Hyperoxaluria;Urolithiasis;Recurrent stone former;Metabolic stone disease
Abstract Objective: Hyperoxaluria leads to calcium oxalate crystal formation and subsequent urolithiasis. This study aims to analyse the effect of treatment compliance in hyperoxaluria, firstly by analysis of patients with non-primary hyperoxaluria and secondly via systematic review in patients with any hyperoxaluria.Methods: In a retrospective cohort study,adults with non-primary hyperoxaluria managed with dietary counselling in 2013 were enrolled. Twenty-four-hour (24 h) urine collections initially and at 6 months were obtained.Compliance was assessed by self-reported dietary compliance and 24 h urinary volume >2 L. Patients were followed for 24 months. Primary outcomes were urinary oxalate and calcium 24 h load at 6 months,and urolithiasis-related procedural rates at 24 months. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-compatible systematic review of compliance among hyperoxaluric patients was performed.Results: In the cohort study,of 19 eligible patients(4 female)with median age 52 years,10(53%)were considered compliant.Compared with the non-compliant group,these patients had significantly increased subsequent 24 h urinary volume (2250 mL vs. 1600 mL; p = 0.008) and lower procedural rates (10% vs. 56%; p = 0.033). Subsequent 24 h urinary oxalate load was nonsignificantly lower in compliant patients. Systematic review regarding compliance in hyperoxaluric patients revealed five studies. Only one utilised dietary counselling or analysed compliant vs. non-compliant patients, finding no difference. None examined the effect of compliance on procedural rates.Conclusion: Hyperoxaluria is an important cause of recurrent urolithiasis.Increasing fluid intake and reducing dietary oxalate reduce the risk of operative intervention and remain fundamental to the treatment of hyperoxaluria.
1. Introduction
Hyperoxaluria is common founding in patients with recurrent calcium oxalate stones [1]. Urinary oxalate is derived from the diet,endogenous production and a small amount comes from the metabolism of ascorbic acid. Normally 1%-15%of dietary oxalate is absorbed, but the amount absorbed depends upon the ingested load of both oxalate and calcium, the abundance of oxalate-degrading intestinal organisms, intestinal epithelial transport characteristics and gut transit time[2-6].Oxalobacter formigenes plays a key role,with stone formers having lower colonisation rates [7-9].Separate to ingestion,oxalate synthesis occurs principally in the liver,utilising amino acids as well as carbohydrates[10].Ascorbic acid breakdown represents the third and final source of urinary oxalate, with the high intake of vitamin C being lithogenic risk factor [11,12].
In humans, oxalate is almost entirely excreted from the proximal tubule, with only a small amount excreted in faeces. In urine, oxalate forms a soluble salt with sodium and potassium. Excessive urinary oxalate excretion supersaturates the urine, leading to nucleation and aggregation and the formation of insoluble calcium oxalate stone crystals [1,13]. Urinary oxalate is the single strongest chemical promoter of kidney stone formation.Compared to calcium,it is estimated to be 15-20 times more potent in promoting stone formation [14]. Four main types of hyperoxaluria are described, namely primary (congenital) hyperoxaluria, and three non-primary entities. These are enteric hyperoxaluria, dietary hyperoxaluria and idiopathic or mild metabolic hyperoxaluria [14,15]. The corner stone to the management of recurrent stone formers with non-primary hyperoxaluria is the dietary restriction of oxalate containing foods in combination with increased fluid intake,with or without calcium supplementation [16,17].
This study aims to assess the compliance of patients with non-primary hyperoxaluria to dietary and fluid advice, and the effect of compliance on subsequent urinary oxalate load and the rate of operative intervention. Additionally, a systematic review will be performed on the effect of patient compliance in hyperoxaluria, to establish this work’s place in the literature.
2. Methods
2.1. Retrospective cohort study
A retrospective analysis of consecutive adult patients managed at a tertiary referral urology centre who received a new diagnosis of non-primary hyperoxaluria based on initial 24 h urine collections was performed. Data were collected from 1st January to 31st December 2013. Nonprimary hyperoxaluria was defined as a urinary oxalate load greater than 0.45 mmol/L per 24 h. Exclusion criteria were features suggesting primary hyperoxaluria. These included a prior such diagnosis, elevated urinary glycolate or L-glyceric acid, suggestive of primary hyperoxaluria type 1 and 2 respectively,and the presence of urolithiasis when of paediatric but not adult age, common in primary hyperoxaluria type 3.
After assessment, all patients were given written information on oxalate containing foods, and instructed to reduce their intake of these items, specifically rhubarb,dark leafy green vegetables, beet, wheat bran, nuts, soy products, tea, chocolate and strawberries [6]. They were also referred to a dietician to assist with understanding and implementing this change. Additionally, all patients were advised to increase fluid intake to >2.5 L per day. Patients were also commenced on thiazide diuretics if they also had concurrent hypercalciuria, provided this was safe in the context of the patient’s other medications and comorbidities.This consisted of oral bendrofluazide 2.5 mg daily.Six months post diagnosis, patients then underwent a second 24 h urinary collection to assess if the interventions altered their urinary biochemical abnormalities.
Patients were followed for a total of 24 months from first clinic review, to assess the relationship between intervention compliance and the need for operative management.After 6 months, no further 24 h urine collections were performed. The authors hypothesised that greater compliance would decrease procedural rates. Primary outcomes were 24 h urinary oxalate load and total volume at 6 months, and rates of operative intervention at 24 months.Treatment compliance was considered present when patients both self-reported dietary compliance,and displayed 24 h urine volume at 6 months exceeding 2000 mL. For the small sub-group prescribed thiazide diuretic medication,compliance was based on patient self-reporting.
Ethics approval was granted by the Austin Research Ethics Committee, reference LNR/16/Austin/555. Unless otherwise stated, data were represented as median followed by interquartile range (IQR) and n represented the number of patients included in the analysis. Continuous data were assessed using the Wilcoxon (Mann-Whitney)test.Categorical measures were summarized as proportions and assessed with Pearson’s Chi-square test. Calculations were performed using STATA version 14 (STATA Corp, College Station, TX, USA). All tests were two-tailed and significance was assessed at the 5% alpha level.
2.2. Systematic review
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [18]. Criteria for considering studies for this review were:Studies published in English in a peer-reviewed journal. Inclusion criteria were, adults with hyperoxaluria of any type with a history of at least one intervention of any type for renal or ureteric uroliathiasis.Primary outcome is patient compliance with intervention(s).The following electronic databases were explored:Cochrane Central Register of Controlled Trials (CENTRAL) (1 January 1946-30 June 2017),EMBASE(1 January 1974-30 June 2017)and MEDLINE (1 January 1946-30 June 2017). Searches utilised the Boolean operators as follows:(OR items#1.#2)AND(OR items #3. #4), searching for terms within abstracts of English language studies, with items of 1. hyperoxaluria, 2.hyperoxaluric, 3. compliance and 4. compliant.
2.3. Data collection and analysis
Eligible studies measured the compliance of patients with hyperoxaluria to a specified intervention.Two independent authors screened the titles and abstracts of identified studies, and selected those that met the pre-defined inclusion criteria.Uncertainty was resolved with discussion.A flow-chart was planned to illustrate sequential screening of identified titles.
2.4. Data extraction
Two authors extracted data onto a pre-designed extraction form. Data were recorded regarding: Author, year of publication, journal, language, country, study design and methodology, population, follow-up duration, compliance rates and other outcomes. The authors expected to find only a small number of studies of small sample size.Hence,no assessment of risk of bias will be performed.
2.5. Data synthesis
Studies were qualitatively summarized, including their methods, interventions and outcomes. The authors did not expect the identified studies will support either quantitative or sub-group analyses.
3. Results
3.1. Retrospective cohort study
3.1.1. Patient characteristics
Nineteen patients with non-primary hyperoxaluria met eligibility criteria.Four(21%)patients were female.Median age was 52 years, with range 23-75 years. Fourteen (74%)patients had lithogenic comorbidities. All patients had previously undergone operative management for urolithiasis. Stone analysis was available on 15 (79%) patients, with all containing calcium oxalate, with or without other salts.Further patient characteristics and stone data are presented in Table 1.
3.1.2. First 24 h urine collection indications and results
The cohort displayed a variety of indications for initial diagnostic 24 h urine collection. These were recurrent urolithiasis in seven (36%) patients, urolithiasis development before 18 years of age in four (21%), lithogenic medical disorder with symptomatic urolithiasis in four(21%), family history of metabolic renal stones in two(11%) and bilateral renal stones in another two cases(11%). For the whole cohort, median initial 24 h urine output and urinary oxalate loads were 1550 mL and 0.54 mmol/L, respectively. Four (21%) patients had hyperoxaluria in association with hypercalciuria, among whom only one had hypercalcaemia. Data are presented in Table 2.
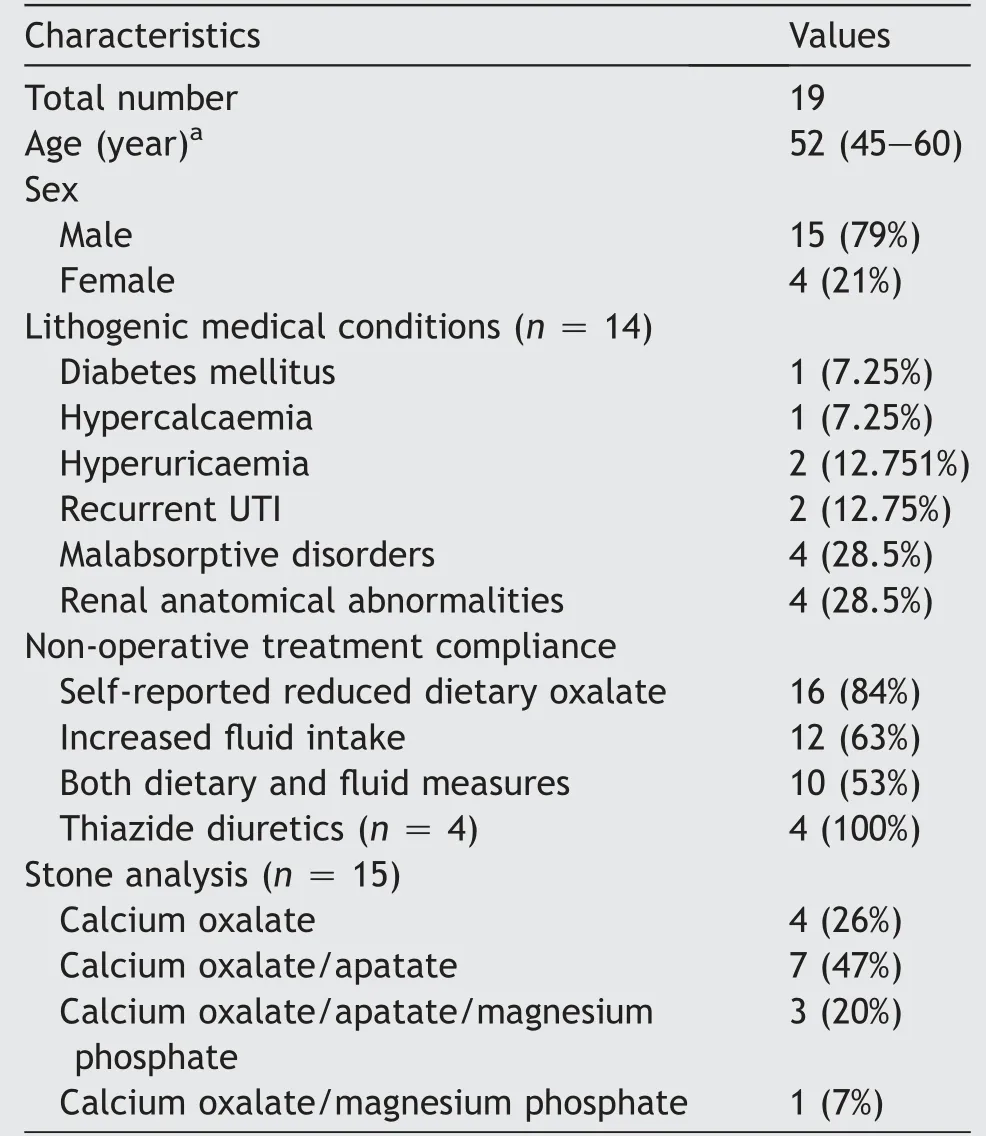
Table 1 Patient characteristics.
3.2. Treatment compliance
Twelve patients (63%) were compliant with increased fluid intake, with subsequent 24 h urine output of >2000 mL.Sixteen patients (84%) self-reported successful dietary modification. From these two groups, 10 patients (53%)were co-operative with both fluid and dietary measures,and were considered “compliant” for subsequent analysis.The remaining nine patients comprised the “noncompliant” group. The four patients prescribed thiazide diuretics reported compliance with their medication.
3.2.1. Impact of dietary oxalate reduction and fluid increase on urinary parameters
The initial 24 h urine parameters of the compliant and noncompliant groups were not statistically significantly different. This included total urine volume, and median urinary loads for oxalate, citrate and calcium. At subsequent 24 h urine collection after 6 months, median total urine volume was significantly greater in the compliant group, at 2250 mL vs. 1600 mL (p = 0.008). Additionally,median oxalate load was lower among compliant patients,at 0.41 nmol/L vs.0.53 nmol/L,however this did not reach statistical significance (p = 0.066). Median urinary citrate and calcium loads were similar. See Table 3 and Fig. 1 for further details.
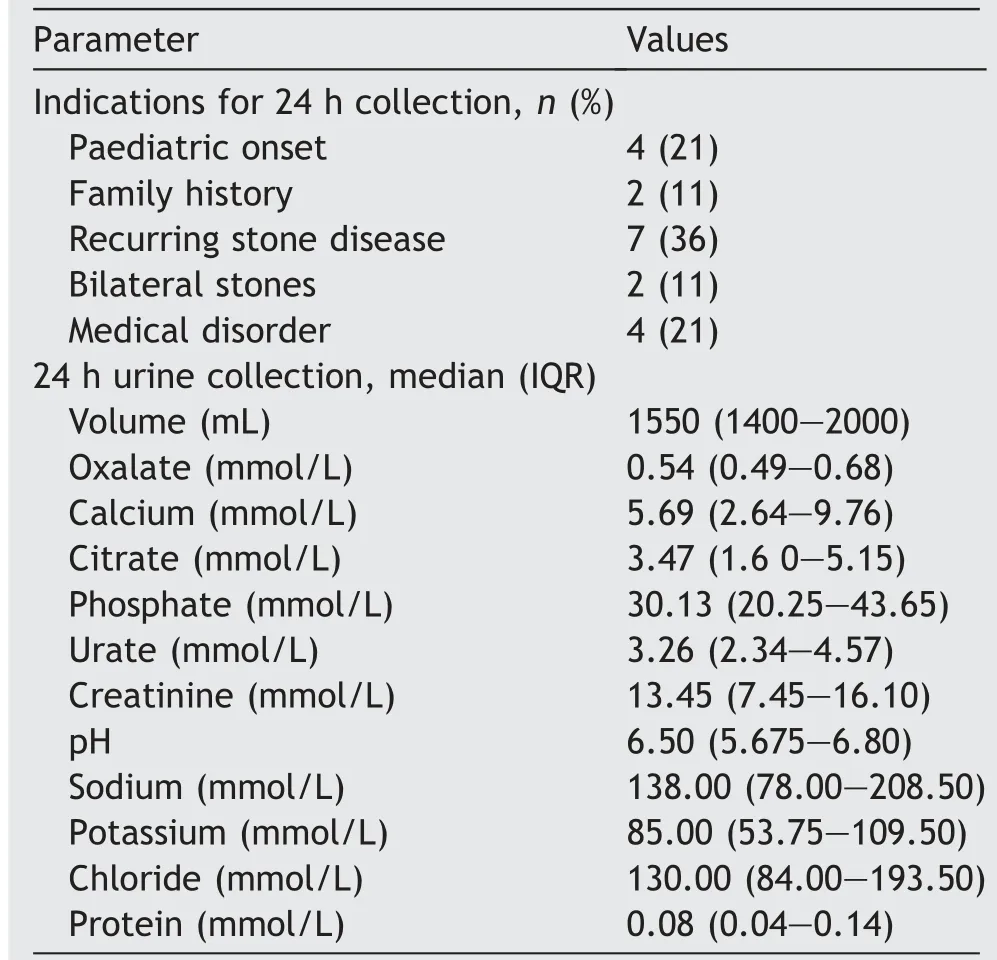
Table 2 First 24 h collection indications and findings.
Among the four patients commenced on thiazide diuretics, there was no significant change in the median 24 h urinary load of either oxalate, at 0.61 mmol/L (IQR:0.50-0.77) vs. 0.52 mmol/L (IQR: 0.45-0.54) (p = 0.195),nor calcium, at 12.64 mmol/L (IQR: 10.20-13.83) vs.8.5 mmol/L (IQR: 7.2-10.1) (p = 0.059).
3.2.2. Impact of dietary oxalate reduction and fluid increase on need for stone treatment
Operative intervention for urolithiasis at 24 months among the compliant and non-compliant cohorts had occurred in 1/10 (10%) and 5/9 (56%) patients, respectively. This difference was statistically significant, with p = 0.033.
3.3. Systematic review
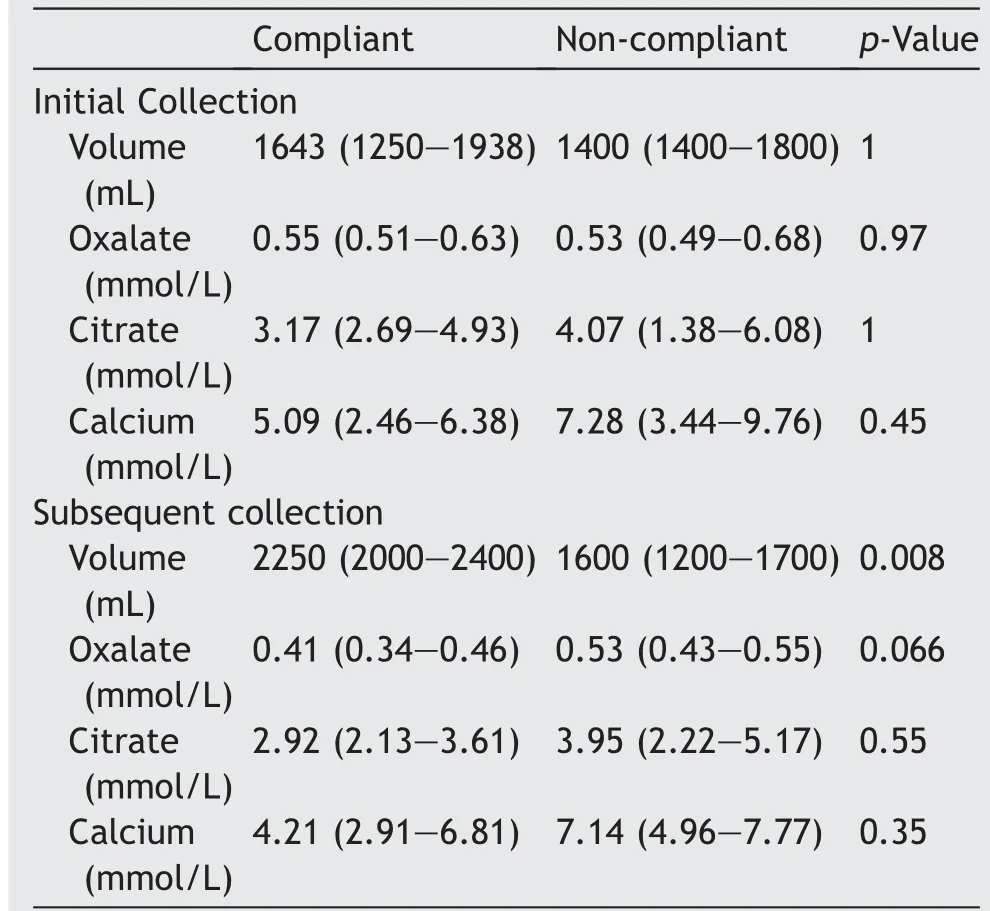
Table 3 Comparison of initial and subsequent 24 h urine collection results between compliant and non-compliant groups, median (IQR).
3.3.1. Citation review
Searches identified 49 potential titles and were view of study titles and abstracts excluded 44 studies for the following reasons: Duplication (19), conference abstract only (11), review articles with no original patients (6) and irrelevant (8). Full text articles of the remaining five articles were retrieved and interrogated against the inclusion criteria. No further studies were eliminated from analysis.See Fig. 3 for systematic review flow chart and Table 4 for eligible study details.
3.3.2. Qualitative analysis
The eligible five studies were diverse in their geographical site, patient age and hyperoxaluric type, intervention and results [19-23]. Only one study compared the urinary oxalate load of compliant and non-compliant patients. Schwen et al. [19] conducted a single centre retrospective cohort study of adults with idiopathic hyperoxaluria with renal stones and at least two 24 h urine collections >30 days apart who were managed with dietary counselling. Compliance was measured by self-reporting, improvement in urine volume and patient ability to keep follow-up appointments.Among 149 eligible patients, 132 (89%) were compliant.Mean urinary oxalate significantly reduced among both compliant and non-compliant patients, with no significant difference between these two groups.
Hoppe et al.[20]conducted a multi-centre double-blind randomised controlled trial(RCT),administering placebo or oral O.formigenes(107colony forming units)twice daily for 24 weeks, with compliance self-reported. Overall, no difference vs.placebo was seen in mean urinary oxalate load.Thirty-seven of 42 enrolled patients (88%) were compliant,however no comparison was performed between these and non-compliant patients.
The remaining three eligible studies were all single centre prospective interventional studies of fifteen patients or less.Only Edwards and Rose[22]utilised a control arm,in their comparison of healthy subjects vs.15 patients with primary hyperoxaluria or mild metabolic hyperoxaluria, receiving various doses of oral pyridoxine.Compared to similarly medicate healthy patients, serum pyridoxal phosphate levels were unchanged in patients with primary hyperoxaluria but significantly lower in those with mild metabolic hyperoxaluria. As measured by urinary 4-pyridoxic acid, all patients were compliant, and thus no comparison against non-compliance was possible.D’Cruz et al. [23] similarly observed 100% compliance to their regime of oral allopurinol 300 mg daily for 2 weeks among adults with enteric hyperoxaluria,as measured by a decrease in plasma uric acid, with no analysis possible against a non-compliant group. Mean urinary oxalate load was unchanged in this eight-patient study.
Conversely, Leumann et al. [21] identified both a significant reduction in mean urinary oxalate load, and noncompliant patients. Seven paediatric patients with primary hyperoxaluria received oral citrate 0.10-0.15 g/kg daily for mean duration 47 months. Two patients were lost to follow up and three (60%) of the remainder were compliant, as measured by variability of urinary pH and citrate load.Compliant patients were seen to enjoy greater long-term renal function. However, their urinary oxalate loads were not compared.
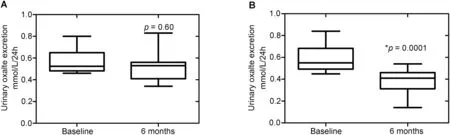
Figure 1 Impact of compliance on 24 h urinary oxalate excretion. (A) Non complaint (n = 9); (B) Complaint patients (n = 10).
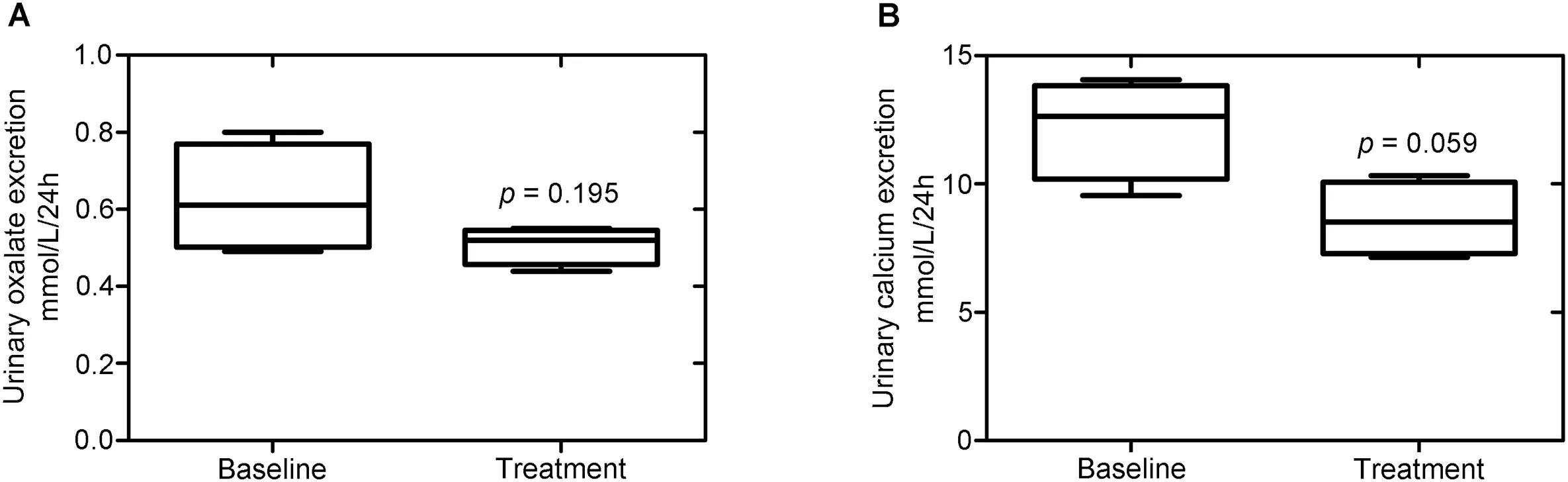
Figure 2 Effect at 6 months of thiazide diuretics on 24 h urinary oxalate and calcium excretion. (A) Urinary oxalate 24 h excretion; (B) Urinary calcium 24 h excretion.
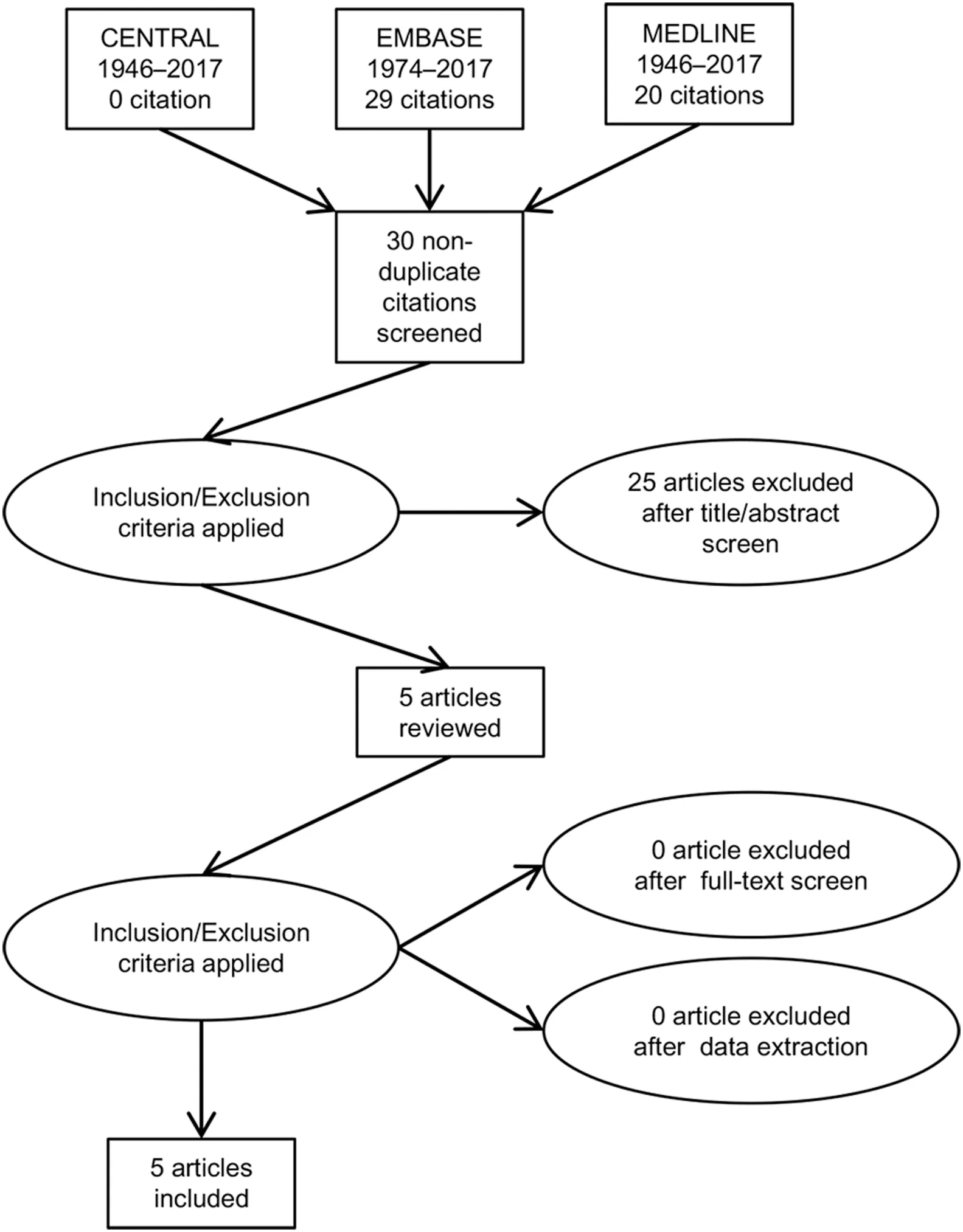
Figure 3 PRISMA flow diagram of citations reviewed in the course of systematic review of compliance to intervention in patients with hyperoxaluria. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
4. Discussion
4.1. Retrospective cohort study
Hyperoxaluria is present in 20%-30% of patients with recurrent calcium oxalate stones. It is the second most common urinary abnormality in recurrent stone formers after hypercalciuria[1].However,excess urinary oxalate is a much stronger promotor of urinary stone formation[24,25]. In this study of patients with non-primary hyperoxaluria, compliance with education to reduce dietary oxalates and increase fluid intake led to significantly greater 24 h urinary volume at 6 months, and lower intervention rates. Twenty-four hour urinary oxalate loads at 6 months were also lower, albeit non-significantly.
This study supported the current literature regarding stone prevention strategies in recurrent stone formers[17,26-28]. Dietary oxalate reduction is essential, and asurinary oxalate excretion may rise up to 80% on high oxalate diets [19,29]. Patients should be advised to avoid rhubarb, dark leafy green vegetables, beet, wheat bran,nuts, soy products, tea, chocolate and strawberries [17]. If consumption of foods containing oxalates will occur, patients should consume additional fluids and also dietary sources of calcium to diminish oxalate absorption. Vitamin C supplements may increase hyperoxaluria and be associated with an increased risk of stone formation. Therefore,the dose of these supplements should be limited to less than 1000 mg per day [30].
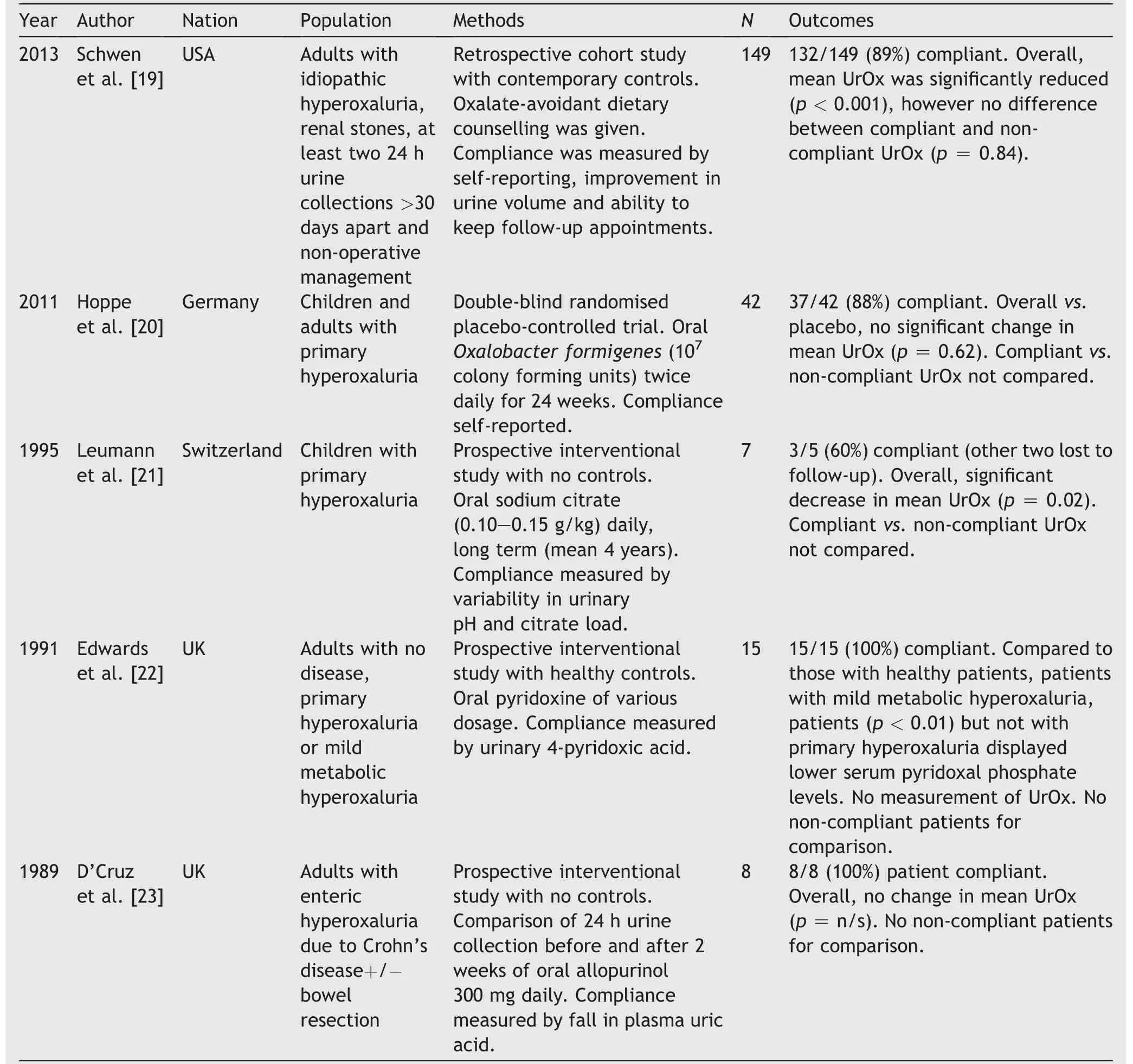
Table 4 Eligible studies resulting from systematic review of intervention compliance among patients with hyperoxaluria.
Patient compliance to interventions is a major issue that affects both medical and surgical fields [31,32]. In this cohort, compliance to medication, dietary measures and high fluid intake were 100%, 84% and 63%, respectively.Only 10 of 19 patients were compliant with both dietary and fluid measures. Current literature regarding compliance in hyperoxaluric patients is sparse and heterogeneous. There were two prospective interventional studies, one retrospective work and one well designed RCT, comprising 221 patients combined. The majority of reported patients are cooperative, with compliance to intervention among patients with hyperoxaluria ranging from 60% to 100%. These results are consistent with our cohort’s findings above, of 63%-100% compliance for three different interventions. To date, no study has examined the effect of compliance on procedural rates in hyperoxaluria, and only one investigation has compared the urinary oxalate loads between compliant and non-compliant patients[19].As in our study,Schwen et al.’s [19] intervention consisted of dietary counselling, but contrastingly, these authors found no difference between compliant and non-compliant patients.
Self-motivation is essential for compliance. The pain of ureteric colic may provide powerful encouragement [33].However, considerable education and coaching may be needed to maintain compliance [27]. Mobile phone applications have shown promise in reducing intake of other dietary salts[33]and may have a role to play in preventing urinary stone disease.
As seen in our study,compliance with medications may be superior to dietary modification [31], with several future therapies currently being investigated. These include supplementation with an oxalate-digesting enzyme, O. formigenes,plant-stem extracts and specific inhibitors of renal tubular oxalate secretion. Oxalobacter is a facultative anaerobic bacteria which digests dietary oxalate, and is present in the large intestine of 70%-80% of adults. However, it is missing or depleted in over 60% of patients with hyperoxaluria[34].Oxalobacter loss can be due to prolonged or repeated antibiotic therapy [35]. Recolonisation with Oxalobacter in adults is difficult. Therefore, attention is currently directed toward developing an Oxalobacterderived oxalate-digesting enzyme therapy [36]. Research is under way involving the transfer of oxalate-digesting enzyme genes from Oxalobacter into a more easily grown bacterium such as Escherichia coli and may eventually provide a new and effective treatment for hyperoxaluria [37].
Other experimental therapies are being investigated and include banana stem juice and beet stem juice. While this appeared to be of benefit in animal studies, to date, no human testing has been performed [38]. L-cysteine has shown some beneficial activity in an animal model,lowering urinary oxalate and calcium load while increasing urinary citrate and magnesium load [39]. Vitamin E supplementation in rats may reduce calcium oxalate crystallization, but the usefulness of this therapy in humans remains unknown [40]. However, none of these experimental agents have become the standard of care for patients with hyperoxaluria.
4.2. Limitations
This is a small single centre retrospective cohort study with heterogeneous contributory lithogenic medical conditions,which limits the generalisability of our results.Additionally,the requirement for satisfaction of both urinary oxalate load reduction and total volume >2 L to be considered“compliant” meant that some “non-compliant” patients were partially compliant,which may have affected results.
5. Conclusion
Hyperoxaluria is a common urinary biochemical abnormality that is a strong risk factor for recurrent urolithiasis.Reducing consumption of oxalate-containing foods and increasing fluid intake remain the cornerstones of treatment. In this retrospective cohort study, hyperoxaluric patients compliant with both interventions were significantly less likely to require operative intervention for urinary stones within 24 months compared to non-compliant patients. Systematic review revealed that this is the first work examining the effect of compliance in hyperoxaluria on urolithiasis-related procedural rates, and only the second to comparing compliant and non-compliant hyperoxaluric patients in any form. These findings reinforce the importance of compliance to dietary advice in the management of non-primary hyperoxaluria.
Author contributions
Study concept and design: Derek B. Hennessey, Gilbert Rice, David Curry.
Data acquisition: Derek B. Hennessey, Gilbert Rice, David Curry.
Drafting of manuscript: Derek B. Hennessey, Ned Kinnear.
Critical revision of the manuscript: Derek B. Hennessey,Ned Kinnear, Siobhan Woolsey, Brian Duggan.
Final approval of manuscript: Derek B. Hennessey, Ned Kinnear,Gilbertc Rice,David Curry,Siobhan Woolsey,Brian Duggan.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2019年2期
Asian Journal of Urology2019年2期
- Asian Journal of Urology的其它文章
- Asynchronous abdominal wall and sigmoid metastases in clear cell renal cell carcinoma:A case report and literature review
- Dose escalation of external beam radiotherapy for high-risk prostate cancer-Impact of multiple high-risk factor
- Effect of warm bladder irrigation fluid for benign prostatic hyperplasia patients on perioperative hypothermia, blood loss and shiver: A meta-analysis
- Is Retzius-sparing robot-assisted radical prostatectomy associated with better functional and oncological outcomes?Literature review and meta-analysis
- Beyond prostate, beyond surgery and beyond urology: The “3Bs” of managing non-neurogenic male lower urinary tract symptoms
- Systemic treatment for metastatic prostate cancer
