Structure-Property Relationship of Light-Responsive Wormlike Micelles Using Methoxycinnamate Derivatives as Light-Switchable Molecules
Tongqing Liu , Fangfang Xue , Ping Yi , Zhiyu Xia , Jinfeng Dong ,*, Xuefeng Li ,*
1 Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, P.R.China.
2 Oil and Gas Technology Research Institute, PetroChina Changqing Oilfield Company, Xi’an 710018, P.R.China.
3 National Engineering Laboratory for Exploration and Development of Low Permeability Oil and Gas Fields,Xi’an 710018, P.R.China.
Abstract: In this work, light-responsive viscoelastic wormlike micelles based on cetyltrimethylammonium hydroxide (CTAOH) and cinnamic acid derivatives, including cinnamic acid (CA), 2-methoxycinnamic acid (2-MCA),3-methoxycinnamic acid (3-MCA), 4-methoxycinnamic acid (4-MCA), 2,3-dimethoxycinnamic acid (2,3-DMCA), 2,4-dimethoxycinnamic acid (2,4-DMCA), 2,3,4-trimethoxycinnamic acid (2,3,4-DMCA), and 3,4,5-trimethoxycinnamic acid (3,4,5-DMCA), were prepared.The effects of the CA derivative structures, especially the position and number of methoxy moieties, on the formation of wormlike micelles were systematically determined.The CA derivatives facilitated the formation of long and entangled wormlike micelles.1H NMR results showed that the CA derivatives participated in the formation of wormlike micelles via insertion of the aromatic moieties into the aggregates.The number of methoxy moieties had a much stronger effect on the viscosity of the wormlike micelle solution than the position of this moiety.The larger the number of methoxy moiety, the smaller was the aggregate.Substituted methoxy moieties increased the steric hindrance between the surfactants and CA molecules, thus hindering the formation of large aggregates.However,the position of the methoxy moiety had a predominant effect on the UV-light-induced transition of the wormlike micelles.Specifically, the ortho-methoxy moiety in the CA molecules dramatically enhanced the efficiency of UV-light-induced transcis isomerization.For example, the 2-MCA/CTAOH, 3-MCA/CTAOH, and 4-MCA/CTAOH binary systems (90 mmol·L-1/100 mmol·L-1) were gel-like with similar viscosities of around 20 Pa·s, but after UV light irradiation, they were transformed into a fluid with lower viscosity because of the formation of smaller aggregates.However, the irradiation time required for the transition varied significantly, as suggested by the results of viscosity measurements and UV-Vis spectroscopy.The 2-MCA/CTAOH system underwent complete phase transition within 3 h, whereas continuous transitions were observed for the 3-MCA/CTAOH and 4-MCA/CTAOH systems upon irradiation for 24 h.1H NMR results suggested that the change in the configuration of MCA in the micelles before and after irradiation was the major cause of the abovementioned difference in the phase transition pattern.Initially, all the aromatic moieties of the trans-2-MCA molecules were deeply inserted into the hydrophobic cores of the micelles in a vertical manner, and the ionized carboxyl moiety was located in the palisade layer because of the electrostatic interactions between CTAOH and trans-2-MCA.In contrast, cis-2-MCA was inserted into the micelles in a horizontal manner, and some of the protons in the aromatic moiety were also transferred from the micellar core to the polar palisade layer.Accordingly, the CTAOH and cis-2-MCA molecules were packed loosely in the aggregates,thereby resulting in the formation of spherical micelles.Similar UV-light-induced transitions were observed for the 3-MCA/CTAOH and 4-MCA/CTAOH systems.
Key Words: Cetyltrimethylammonium hydroxide; Cinnamic acid derivative; Wormlike micelle; Light response;trans-cis isomerization
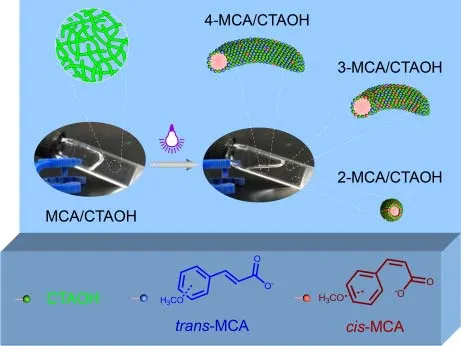
1 lntroduction
The formation of self-organized aggregates with rich morphologies including spherical micelles, wormlike micelles and vesicles is one of the major characteristics of surfactants in the bulk phases1-3.Those aggregates also endow surfactants with the wide application functions such as drug delivery,solubilization, templates of nanomaterials, and so forth4-7.In the past decades, the developed stimuli-responsive surfactant system to numerous environmental factors including pH,temperature, light, and redox were attracted much attention because of their promising potential in applications8-11.As a stimulus factor, light is non-invasive to the sample that can be realized conveniently.Accordingly, the light-response of surfactant systems was studied mostly12.
Generally, the introduction of particular light-responsive moieties such as spiropyran, azobenzene, stilbenzene, and coumaric moieties in surfactant systems by either covalent or non-covalent route can endow them with interesting lightresponse13-17.For example, Zhao and coworkers18developed a Gemini surfactant C12-azo-C12 with light-response by the covalent route, in which the introduction of azobenzene moiety as spacer in the molecule was the major characteristic.It formed wormlike micelles and rodlike micelles in itstrans- andcisisomers, respectively, and the light-induced micellar transition could be realized through irradiating by UV- and visible light reversibly.Based on the non-covalent route, similar lightinduced reversible transition between wormlike micelles and rodlike micelles could also be realized19, in which a lightresponsive hydrotropic salt 1-[2-(4-phenylazo-phenoxy)-ethyl]-3-methylimidazolium bromide (C0AZOC2IMB) was introduced into the sodium oleate (NaOA) aqueous solution.
Cinnamic acid (CA) and its derivatives are a category of lightresponsive chemicals, which are widely employed to develop light-responsive systems by the non-covalent route20-22.Raghavan22reported a simple class of photorheological fluids composed of cetyltrimethylammonium bromide (CTAB) and 2-methoxycinnamic acid (2-MCA).Initially, very long and entangled wormlike micelles were formed, and thereby endowing the system with very large viscosity.However, the sample became a thin fluid after UV-light irradiation because of the formation of rodlike micelles caused by thetrans- tocisisomerization of 2-MCA.Indeed, similar UV-light induced formation of smaller aggregates based on CA derivatives was often observed in both polar and apolar systems, and thetranstocis-isomerization of them was the major cause23,24.Recently,we have reported the interesting UV-light induced multillamellar vesicle to micelle transitions in the mixtures of alkyldimethylamine oxide and coumaric acid, and the position of substituted hydroxy moiety in the CA molecule affected the transition significantly25.Similar effect of substituted moiety in CA molecule on the UV-light induced transition was also observed in the apolar systems26.
In this work, light-responsive viscoelastic wormlike micelles composed of cetyltrimethylammonium hydroxide (CTAOH)and cinnamic acid derivatives (Table 1), including CA,2-methoxycinnamic acid (2-MCA), 3-methoxycinnamic acid (3-MCA), 4-methoxycinnamic acid (4-MCA), 2,3-dimethoxycinnamic acid (2,3-DMCA), 2,4-dimethoxycinnamicacid (2,4-DMCA), 2,3,4-trimethoxycinnamic acid (2,3,4-TMCA) and 3,4,5-trimethoxycinnamic acid (3,4,5-TMCA),were developed and studied by various techniques such as rheology,1H NMR, UV-Vis spectrum and cryogen transmission electron microscopy (cryo-TEM) measurements.In specifically,the effect of different CA derivatives on the self-assembly behaviors of binary systems were studied, and factors including the number and position of substituted-methoxy moiety in CA molecule were considered in detail.Moreover, the effect of substituted-methoxy moiety on the UV-light induced wormlike micelles transitions were also studied systematically.This work reveals the structure-property relationships of light-responsive wormlike micelles using methoxy-CA derivatives (MCA) as the light-switchable molecules and provides very important information at the molecular level, which is fundamental important in colloids and interfaces.
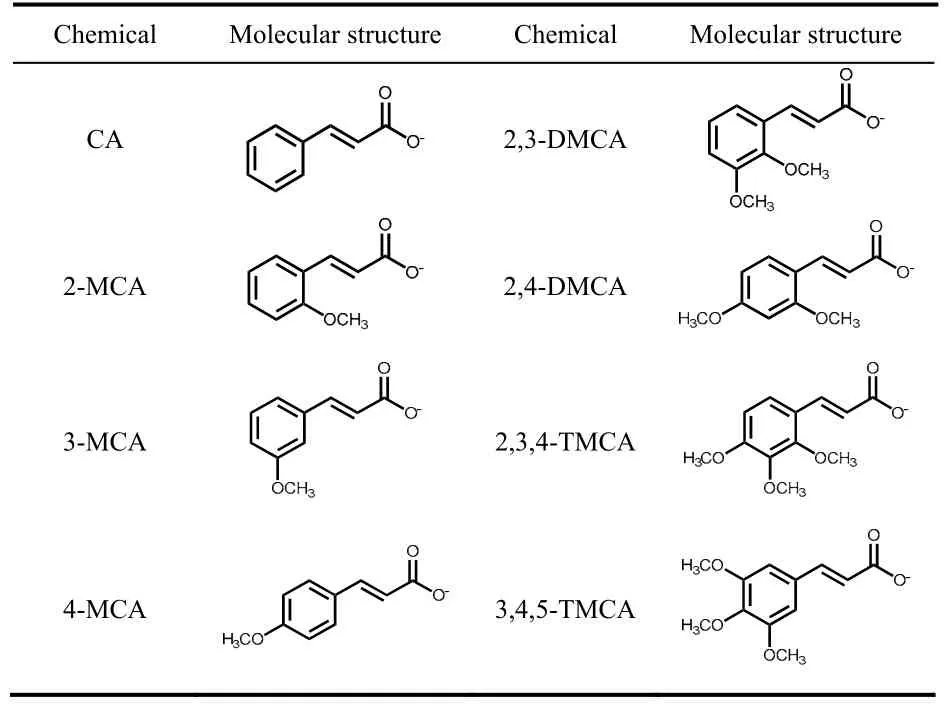
Table 1 Molecular structures of CA derivatives employed.
2 Experimental
2.1 Chemicals
Cetyltrimethylammonium hydroxide (10% in water, TCI) and cinnamic acid derivatives including cinnamic acid (99%, J&K), 2-methoxycinnamic acid (99%, Alfa Aesar), 3-methoxycinnamic acid(98%, Alfa Aesar), 4-methoxycinnamic acid (98%, Alfa Aesar), 2,3-dimethoxycinnamic acid (98%, TCI), 2,4-dimethoxycinnamic acid(98%, Sigma), 2,3,4-trimethoxycinnamic acid (98%, TCI) and 3,4,5-trimethoxycinnamic acid (98%, Alfa Aesar) were used as received.
2.2 Sample preparation
All samples were prepared by dissolving a certain amount of cinnamic acid derivatives in 100 mmol·L-1CTAOH aqueous solution, which were stirred at 80 °C to complete the acid-base neutralization reaction until the samples became homogeneous.And then, the samples were stored at 25 °C at least 48 h before measurements.
2.3 Light irradiation of samples
UV-light irradiation experiments were performed by OPTIMAXTM365 (wavelength 365 nm, Spectronics, USA) with the power of 50 mW·cm-2at 15 cm away.For each irradiation experiment, 3 mL sample was employed and the distance between the sample and light source was fixed at 15 cm at 25 °C.
2.4 UV-Vis spectra measurements
UV-Vis spectra measurements were carried out on UV-Vis Tu-1901 spectrophotometer (Pgeneral, China) using ultrapure deionized water (Millipore) as a blank at 25 °C.
2.5 Rheological measurements
Steady rheological measurements were performed on a RS 600 stress-controlled rheometer (TA Instruments, USA) using a cone plate geometry (diameter 35 mm and cone angle 1°) at 25 °C.Samples were equilibrated for at least 30 min before measurements and a solvent trap was used to minimize sample evaporation.
2.6 1H NMR measurements
1H NMR spectra were recorded on a 400 MHz Bruker-BioSpin spectrometer (Germany) at 25 °C.
2.7 Cryogen transmission electron microscopy measurements
Generally, a drop of sample about 4 μL was dropped on the TEM copper grid covered by a holey carbon film, and the excess liquid was blotting away to form a thin liquid film.Then, the grid was quickly plunged into liquid ethane at its melt temperature.The specimens were maintained at approximately -173 °C and imaged in a transmission electron microscope (Talos, ThermoFisher Scientific, USA) at an accelerating voltage of 200 kV under low dose conditions.
3 Results and discussion
3.1 Effect of CA derivatives on the self-assembly behaviors
It was reported that CTAOH formed spherical micelles in the aqueous solution27, and wormlike micelles and vesicles might also be formed through adding hydrotropic salts28.In the CA derivatives/CTAOH binary systems, all samples were optical transparent and their viscosities were depended on the concentration and type of added CA derivatives dramatically.The macro-appearance suggested that micelles were commonly formed in them based on the electrostatic interaction between CA derivatives and CTAOH molecules.Since spherical micelles and wormlike micelles often show different rheological characteristics, then these systems were further studied by the rheological method.Fig.1a shows the representative static rheological responses of CA/CTAOH, and Fig.1b is the corresponding CA concentration ([CA]) dependent zero-shear viscosity (η0) results.
Obviously, [CA] affected the zero-shear viscosity of samples dramatically.In the presence of 60 and 75 mmol·L-1CA, the sample was a Newtonian fluid with a low viscosity about 1 mP·s,corresponding to the spherical micelles solution.Once [CA] was increased to 80 mmol·L-1, it showed a shear-thinning behavior with the value ofη0about 4 Pa·s.The viscosity can be further enhanced through increasing [CA], and the maximum viscosity about 400 Pa·s was achieved in the region of 100 to 120 mmol·L-1CA.These shear-thinning behaviors are typical rheological characteristic of wormlike micelles29.Instead, the bulk viscosity was decreased conversely upon further increasing[CA],i.e., 130 mmol·L-1, which is often attributed to the formation of branched wormlike micelles or multi-connected micellar networks30-32.
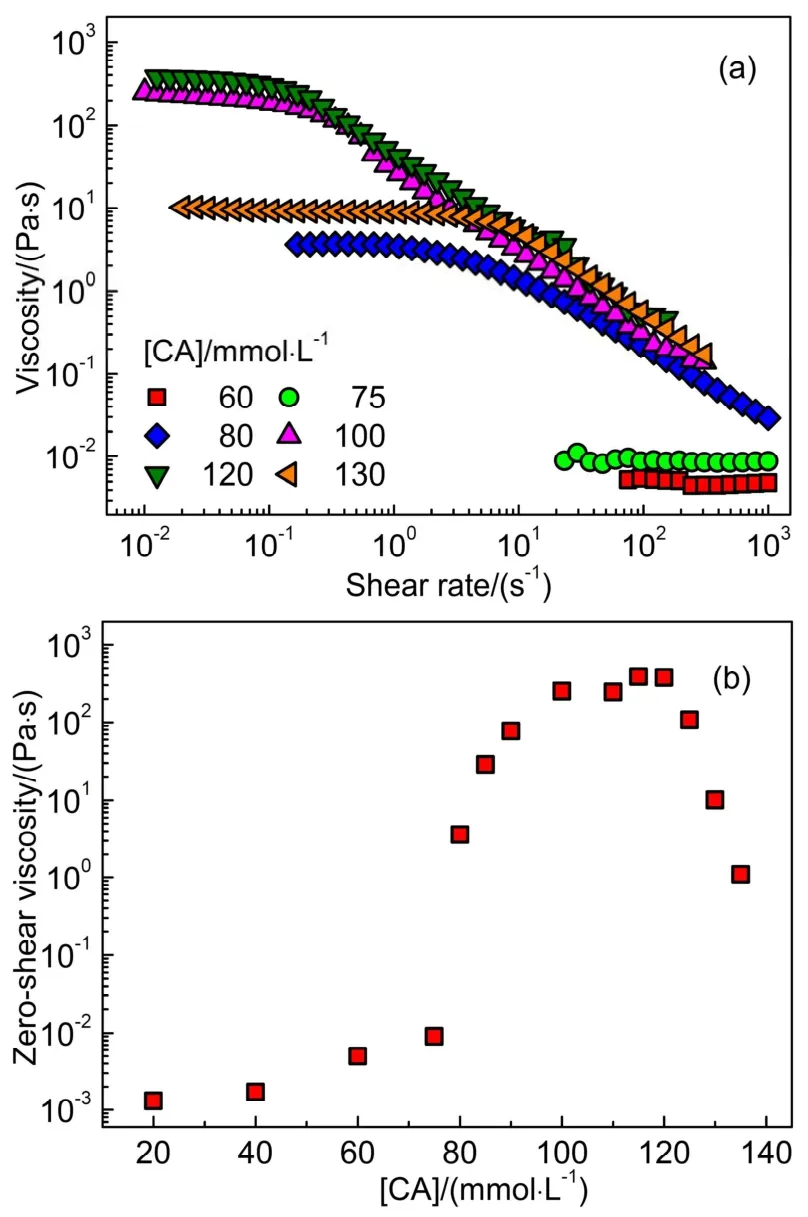
Fig.1 (a) The static rheological responses of the CTAOH/CA binary system in the presence of 100 mmol·L-1 CTAOH with different amount of CA, and (b) the corresponding CA concentration dependent zero-shear viscosity results.
The results indicated the CA-induced micellar growth from spherical to wormlike micelles.Similar concentration-induced rheological responses were also observed in the presence of single-methoxy substituted CA derivatives (MCA) including 2-MCA, 3-MCA and 4-MCA (Fig.2a).The variation tendency of three systems was nearly the same, in which the viscosities were increased about 104times when [MCA] was increased from about 60 to 80 mmol·L-1.The achieved maximum viscosity of CA/CTAOH (Fig.1b) was much higher than those of MCA/CTAOH (Fig.2a), indicating the introduced methoxy moiety in CA molecules was disadvantage to the formation of larger wormlike micelles.The increased steric hindrance between surfactants and hydrotropic salts is the major cause.
Furthermore, the maximum viscosities of MCA/CTAOH systems followed the order of 2-MCA < 3-MCA < 4-MCA,indicating the location of methoxy moiety also affected the micellar microstructure slightly.This regulation became even more intuitive in the poly-methoxy substituted CA derivatives(Fig.2b).For example, 2,4-DMCA was much more efficient than 2,3-DMCA in inducing the growth of micelles because the dramatic increase of viscosity was happened above 60 and 80 mmol·L-1, respectively.In addition, the achieved maximum viscosity of 2,4-DMCA/CTAOH was comparable with that of either 2-MCA/CTAOH or 4-MCA/CTAOH, whereas that of 2,3-DMCA/CTAOH was far smaller than the MCA/CTAOH systems.The results suggest that themeta-substituted methoxy moiety is highly disadvantage to the formation of larger wormlike micelles, whereas theortho- andpara-substituted ones affect a little.

Fig.2 The corresponding CA derivatives concentration dependent zero-shear viscosity of 100 mmol·L-1 CTAOH solutions,(a) and (b) represent the single- and poly-methoxy substituted derivatives of CA, respectively.
Obviously, the increased number of methoxy moieties enlarges the hydrophobic volume of hydrotropic salt, which benefits the growth of micelles.For example, the additionally introducedpara-methoxy moiety increased the hydrophobic volume of hydrotropic salt, resulting in a relatively larger maximum viscosity as observed from the 2,3-DMCA/CTAOH and 2,3,4-TMCA/CTAOH (Fig.2b).Simultaneously, it also increases the steric hindrance between surfactants and hydrotropic salts, which impends the formation of larger aggregates.It should be mentioned that the steric hindrance is the predominant effect factor on the micellar micro-structures.In comparison with 2,4-DMCA/CTAOH and 2,3,4-TMCA/CTAOH(Fig.2b), the latter one had a lower maximum viscosity because the additionalmeta-methoxy moiety increased the steric hindrance significantly.Attributing to the presence of twometamethoxy moieties in 3,4,5-TMCA, only a slight viscosity change was observed regardless of its concentration.Moreover, its maximum viscosity was below 10 mP·s, which was far smaller than those of 4-MCA/CTAOH and 2,3,4-TMCA/CTAOH binary systems.
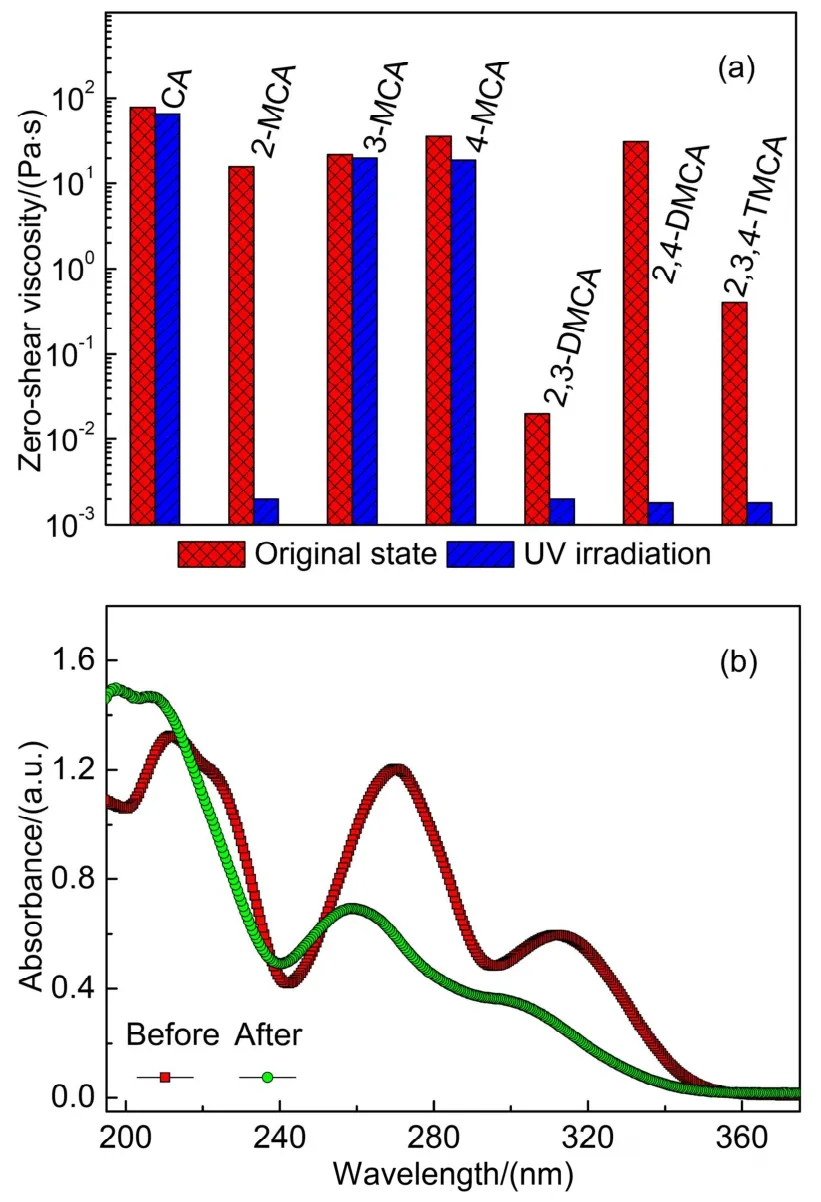
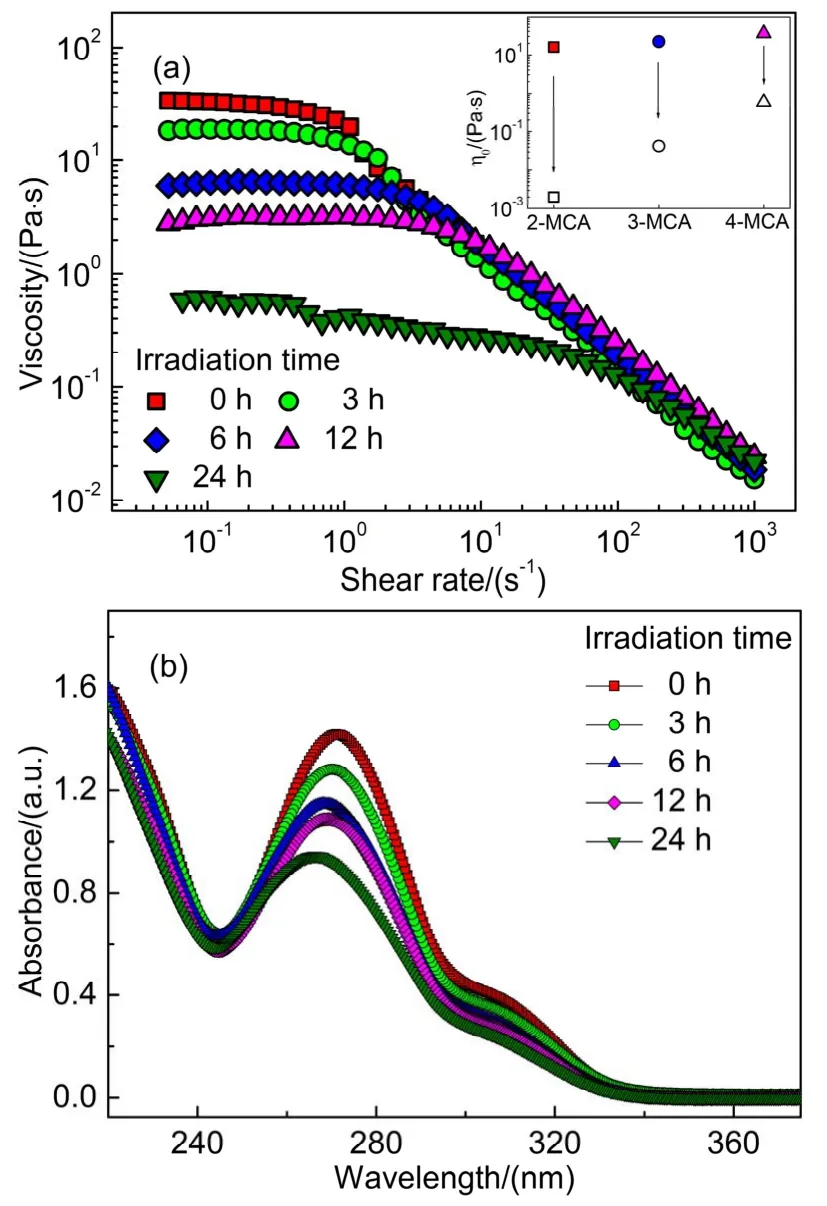
Fig.3a shows the1H NMR spectra of 10 mmol·L-1CTAOH in the presence of 1-7 mmol·L-1CA.The chemical shifts of different protons from both CTAOH and CA molecules were shifted toward upfields gradually upon increasing [CA].Simultaneously, significant broadening of the resonance was also appeared when [CA] was above 6 mmol·L-1, indicating the formation of larger aggregates33-35.Fig.3b shows the corresponding[CA]-dependent chemical shift changes Δδ(Δδ=δbinarymixture-δpure)of CA.Obviously, all Δδvalues from Hato Hfwere negative and became more negative with the increased [CA].The results suggest that CA molecules are embedded into the micellar core and participated in the formation of micelles19,36.Moreover, the absolute Δδvalues from Hato Hffollowed the order of Ha≈ Hb Similar changes were also observed in other CA derivatives/CTAOH systems, and the [CA derivatives]-dependent chemical shift changes Δδwere studied (Fig.3).Three protons including the proton Hcpresented in all CA derivatives (signed by the same manner as CA molecule in Fig.3a), protons Hαand Hβin CTAOH molecule were employed as shown in Fig.3c-e,respectively.Except the protons of the 3,4,5-TMCA/CTAOH binary system, Δδvalues of Hc, Hαand Hβof others were all negative that were decreased upon increasing [CA derivatives].The negative Δδvalues of Hcindicated the aromatic moieties of CA derivatives were also embedded into the micellar core and participated in the formation of micelles efficiently.Instead, the Δδvalues around 0 indicated the weak interaction between 3,4,5-TMCA and CTAOH molecules because of the strong steric hindrance between them.Thus,1H NMR results evidently confirm the general mechanism of CA and its derivatives in inducing the micellar growth. Fig.3 (a) 1H NMR spectra of 10 mmol·L-1 CTAOH with different amount of CA and the corresponding [CA derivatives]-dependent chemical shift change of particular protons in systems (b–e).(b) different protons in CA molecule, (c) proton Hc in different CA derivatives, protons of Hα (d) and Hβ (e) of CTAOH, respectively. It is well-known that the UV-light induced morphological transitions of aggregates in CA and its derivatives containing systems are caused by thetrans-cisisomerization of them37.In order to clarify the effect of the molecular structures of CA derivatives on the UV-light induced morphological transitions,the particular composition of 90 mmol·L-1CA derivatives/100 mmol·L-1CTAOH was employed.In this composition, the approximately maximum viscosity was arrived for each system.Fig.4 shows the corresponding viscosity variation of samples before and after UV-light irradiation for 3 h, and a clear tendency can be observed. The viscosities of 2-MCA/CTAOH, 2,3-DMCA/CTAOH,2,4-DMCA/CTAOH and 2,3,4-TMCA/CTAOH were decreased dramatically after UV-light irradiation, whereas those of CA/CTAOH, 3-MCA/CTAOH and 4-MCA/CTAOH were only weakened slightly.Anyway, the results suggested that UV-light irradiation induced viscosity decrease was commonly presented in these systems.UV-Vis spectra of 2-MCA/CTAOH showed the remarkable absorbance change of 2-MCA before and after irradiation (Fig.4b).In specifically, the absorbance intensity was decreased with the apparent blue-shift of absorbance peak.That evidently confirmed the UV-light inducedtrans-cisisomerization of 2-MCA molecules.Thus, the UV-light induced thinning behaviors are attributed to thetrans-cisisomerization of CA and its derivatives as reported previously37,38.More importantly, the presence of methoxy moiety in theorthoposition of CA molecule seemed to be critical in facilitating viscosity decrease.That’s to say, it dramatically enhanced the UV-light inducedtrans-cisisomerization efficiency.Otherwise,the UV-light induced viscosity decrease became a timeconsuming process as observed in the CA, 3-MCA and 4-MCA containing systems (Fig.4a).Similar observation was also reported previously, in which the weakened light penetration to the interior regions impeded by wormlike micelles was considered to be the major cause39. Fig.4 (a) Viscosity of CA derivatives/CTAOH binary systems before and after UV-light irradiation for 3 h, and (b) UV-Vis spectra of 2-MCA/CTAOH diluted 1000 times before and after irradiation. In order to understand the importance of the position of substituted methoxy moiety, three samples of 2-MCA/CTAOH,3-MCA/CTAOH and 4-MCA/CTAOH were studied further.These samples showed the similar viscosity around 20 Pa·s,which were continuously irradiated for 24 h.Fig.5a shows the corresponding rheological responses of 4-MCA/CTAOH system.The viscosity was decreased gradually upon increasing the irradiation time, and similar tendency was also observed in either 2-MCA/CTAOH or 3-MCA/CTAOH system.It was noticed that the viscosity of irradiated MCA/CTAOH sample was highly depended on the position of methoxy moiety (the insert image in Fig.5a), which followed the order of 2-MCA/CTAOH < 3-MCA/CTAOH < 4-MCA/CTAOH.The irradiation timedependent UV-Vis spectra of 3-MCA/CTAOH (Fig.5b) showed that the absorbance intensity of 3-MCA was decreased within 24 h, confirming the time-consuming UV-light inducedtrans-cisisomerization. Fig.5 (a) Viscosity of 4-MCA/CTAOH and (b) UV-Vis spectra of 3-MCA/CTAOH diluted 1000 times after UV-light irradiation,respectively.The insert image represents the zero-shear viscosity variation of MCA/CTAOH by UV-light irradiation. Therefore, it can be concluded that the time-consumingtranscisisomerizations of 3-MCA and 4-MCA were not caused by the hindrance to UV-light from aggregates39, because wormlike micelles with similar micro-structures were formed in the studied three samples.Alternatively, the methoxy moiety induced configuration variation in MCA molecules might be the major cause.Theortho-methoxy moiety in 2-MCA molecule breaks the conjugated system between the aromatic ring and ethylene bond, attributing to the repulsive interaction between methoxy moiety and propenyl C―H40.As a result, theorthomethoxy moiety facilitated thetrans-cisisomerization of the ethylene bond, and thereby exhibiting a relatively rapid UV-light induced thinning behavior in the 2-MCA/CTAOH system.Instead, the UV-light inducedtrans-cisisomerization became less efficient because the conjugated system in 3-MCA and 4-MCA molecules were remained.Similar observation was also reported previously in the CA based light-responsive systems39,41. Fig.6a shows the cryo-TEM image of the original 3-MCA/CTAOH sample.Obviously, very long and entangled wormlike micelles were formed in this system.After UV-light irradiation for 24 h, wormlike micelles were still the predominant morphology though short rodlike micelles were also visible (Fig.6b).However, mainly spherical micelles were formed in the irradiated 2-MCA/CTAOH sample (Fig.6c).Thus,the relatively larger viscosity of 3-MCA/CTAOH in comparison with that of 2-MCA/CTAOH was attributed to the long wormlike micelles remained.It is known that thetrans-cisisomerizations of CA derivatives could increase the hydrophilicity of them or solubility in water37.Accordingly, the embedded CA derivatives in wormlike micelles tended to be deviated from the aggregates,favoring the formation of smaller aggregates.During the UV-light induced micro-structural transitions of wormlike micelles,the micro-environment of CA derivatives should also be changed. In order to understand the importance of the position of substituted methoxy moiety, 2-MCA/CTAOH, 3-MCA/CTAOH and 4-MCA/CTAOH were studied by the1H NMR method.Fig.7 shows the typical1H NMR spectra of 10 mmol·L-1CTAOH in the presence of 1-7 mmol·L-12-MCA before and after UV-light irradiation.Before UV-light irradiation (Fig.7a), the1H NMR spectra of 2-MCA/CTAOH showed the same variation tendency as those of CA/CTAOH (Fig.3), indicating 2-MCA played the same function as that of CA in inducing the growth of CTAOH micelles.Once the samples were irradiated by UV-light (Fig.7b),trans-2-MCA was isomerized intocis-2-MCA.Accordingly,peaks in the1H NMR spectra of samples containing 6 and 7 mmol·L-12-MCA became distinguishable again, suggesting the formation of smaller aggregates. The [2-MCA]-dependent chemical shift changes Δδof protons in the aromatic moieties were shown in Fig.7c.The Δδvalues of all protons in the box were negative, which were decreased gradually with the increase of [2-MCA] in the original samples.That suggested the aromatic moiety oftrans-2-MCA was changed from the original polar to an apolar microenvironment19,36.In other words,trans-2-MCA was participated in the formation of wormlike micelles through inserting its aromatic ring into the micellar core.Moreover, protons Heand Hfwere inserted deeper because of the relatively larger Δδvalues.Once the samples were irradiated by UV-light, the Δδvalue of each proton was kept nearly constant regardless of [2-MCA].That indicatedcis-2-MCA molecules should be located in the same micro-environmental condition, which can be attributed to the commonly formed spherical micelles after UV-light irradiation (Fig.6c).The Δδvalues of protons Hd, Heand Hfincis-2-MCA molecules were still negative, whereas that of Hcbecame positive.The positive Δδvalue of Hcindicated that it was located in an even more polar micro-environment than the purecis-2-MCA in water.Therefore, it should be located in the palisade layer of micelles nearby the charged interface composed of CTAOH ionic headgroup34,36.The negative Δδvalues of Hd,Heand Hfindicatedcis-2-MCA molecules were still participated in the formation of mixed micelles.Except the absolute Δδvalue of proton Hfwas a relatively large, those of Hdand Hebecame much smaller after UV-light irradiation, suggesting they should be transferred into more polar environment instead.That’s to say,trans-2-MCA was inserted deeper thancis-2-MCA in micelles. Fig.6 Cryo-TEM images of original 3-MCA/CTAOH (a), and UV-light irradiated samples of 3-MCA/CTAOH (b) and 2-MCA/CTAOH (c), respectively.Bars represent 100 nm. Fig.7 1H NMR spectra of 10 mmol·L-1 CTAOH with different amount of 2-MCA before (a) and after (b) UV irradiation for 3 h, and the concentration of pure 2-MCA sample was 10 mmol·L-1.(c) and (d) represent the corresponding [MCA]-dependent chemical shift change of particular protons before (solid) and after (hollow) UV irradiation, respectively. Simultaneously, the configuration of inserted 2-MCA molecules was also changed after UV-light irradiation.Initially,the whole aromatic moiety oftrans-2-MCA molecule was inserted into the hydrophobic micellar core with a vertical manner, and its ionized carboxyl moiety was located in the palisade layer based on the electrostatic interaction between CTAOH andtrans-2-MCA.After UV-light irradiation,cis-2-MCA was inserted into micelle by a horizontal manner instead,in which the proton Hcwas transferred from the micellar core to the polar palisade layer.Accordingly, spherical micelles were formed because CTAOH andcis-2-MCA molecules adopted the loosely packed manner in micelles.Fig.7d shows the corresponding results of two typical protons Hcand Hdof 3-MCA and 4-MCA before and after UV-light irradiation, and similar tendency as that of 2-MCA can be observed.The results suggested that bothcis-3-MCA andcis-4-MCA molecules in aggregates adopted the same configuration as that ofcis-2-MCA,and thereby resulting in the formation of smaller micelles.Therefore, it can be conclude safely that UV-light irradiation induced thinning behaviors in MCA/CTAOH wormlike micelles are caused by the same mechanism as mentioned above. In summary, this work studied the effect of methoxysubstituted CA derivatives on the self-assembly behaviors of CTAOH aqueous solutions before and after UV-light irradiation systematically by various techniques.In these binary systems,CA derivatives commonly facilitated the micellar growth as the hydrotropic salts.Both the position and number of methoxy moieties in CA molecules affected the micro-structures of micelles, whereas the effect of the latter one was much stronger because of the strengthened steric hindrance.Generally speaking,the larger the number is, the smaller the aggregates are.1H NMR results suggested that CA derivatives were participated in the formation of micelles through inserting the aromatic moieties into the hydrophobic micellar cores. The UV-light induced micellar transitions were commonly observed in these systems, owing to thetrans-cisisomerization of CA derivatives.However, the processes were highly depended on the position of methoxy moiety in CA molecules.It was noticed that the presence ofortho-methoxy moiety in CA molecule facilitated thetrans-cisisomerization significantly.For example, the wormlike micelles to spherical micelles transition of 2-MCA/CTAOH could be completed within 3 h, whereas the time-consuming and continuous transitions were observed within the studied 24 h in both 3-MCA/CTAOH and 4-MCA/CTAOH.From the viewpoints of molecular structures, the repulsive interaction betweenortho-OCH3and propenyl C―H facilitates thetrans-cisisomerization of ethylene bond, because it breaks the conjugated system between the aromatic ring and ethylene bond of CA molecule.This work clarifies the structureproperty relationship of light-responsive CA derivatives/CTAOH systems systematically, which is fundamental importance in effectively designing new responsive surfactant systems as guidance.3.2 Effect of CA derivatives on UV-light response



4 Conclusions
- 物理化學(xué)學(xué)報(bào)的其它文章
- 基于兩親性喹喔啉的超分子凝膠:手性信號(hào)反轉(zhuǎn)以及多重響應(yīng)手性光學(xué)開(kāi)關(guān)
- Solvent-Induced Inversion of Pickering Emulsions for In Situ Recycling of Enzyme Biocatalysts
- 環(huán)糊精與表面活性劑主客體作用誘導(dǎo)的金納米棒可控自組裝
- Wormlike Micelle to Gel Transition Induced by Brij 30 in Ionic Liquid-Type Surfactant Aqueous Solution
- 含有酰胺基或酯基的可降解陽(yáng)離子Gemini表面活性劑在水溶液中的聚集行為
- 基于多酯頭基的“油-二氧化碳兩親分子”設(shè)計(jì)及其助混規(guī)律

