Effects of Operating Conditions on the Catalytic Performance of HZSM-5 Zeolites in n-Pentane Cracking
Hou Xu; Zhao Liu; Ma Zhenzhou; Chen Bochong; Feng Jingyuan; Cui Tingting
(1. School of Chemical Engineering, Changchun University of Technology, Changchun 130012;2. Advanced Institute of Materials Science, Changchun University of Technology, Changchun 130012;3. Department of Chemistry, Tsinghua University, Beijing 100084)
Abstract: In this work, n-pentane catalytic cracking over HZSM-5 zeolites was studied at 650 °C under atmosphere pressure. A particular attention was paid to the measurement of n-pentane conversion, light olefins production, product distribution, coke deposit, etc. Several indexes were defined to evaluate the effects of operating conditions on the catalytic performance of HZSM-5 zeolites. It was found that decreasing the weight hourly space velocity, increasing the reactant partial pressure, and increasing the carrier gas flow rate could inhibit C-H bond breaking and enhance the C-C bond breaking and hydride transfer reactions, leading to reduced alkenes selectivity, which suppressed the formation of external coke and alleviated the deactivation of HZSM-5 zeolites. It was deduced that the catalytic stability of HZSM-5 zeolites was improved at the cost of alkenes selectivity. Compared with decreasing the weight hourly space velocity and increasing the reactant partial pressure, increasing the carrier gas flow rate could enhance the diffusion process and protect alkenes from being consumed in coke formation in order to improve the catalytic stability of HZSM-5 zeolites with less reduction of alkenes selectivity.
Key words: operating conditions, HZSM-5 zeolites, catalytic performance, n-pentane cracking, light olefins
1 Introduction
Light olefins, especially ethylene and propene, are vital for the synthesis of polymer materials and organic chemicals. The continuous progress of living standards puts up a growing demand for ethylene and propene production[1-2]. HZSM-5 zeolites were regarded as the promising candidate catalysts to improve naphtha cracking process and light olefins production[3-7].Operating conditions, e.g. reaction temperature, space time, reactant partial pressure, and carrier gas, made a significant difference on the catalytic performance of HZSM-5 zeolites. Miyaji, et al.[8]found that an increase of reactant partial pressure promoted ethylene selectivity while reducing propene selectivity inn-pentane catalytic cracking. Hodoshima, et al.[9]found that the presence of carrier gas promoted ethylene and propene formation and alleviated catalyst deactivation inn-hexane catalytic cracking. Kim, et al.[10]found that an increase of reaction temperature and the absence of carrier gas accelerated the deactivation of HZSM-5 in JP-8 catalytic cracking.
Epelde, et al.[11-12]found that an increase of reaction temperature, a decrease of space time, and a decrease of reactant partial pressure promoted propene selectivity in the transformation of 1-butene. Roohollahi, et al.[13]found that an increase of reaction temperature and a decrease of contact time promoted the selectivity to light olefins while reducing the selectivity to paraffins and heavy products ini-butane andn-butane catalytic cracking. Cordero-Lanzac,et al.[14-15]found that an increase of reaction temperature promoted alkenes and coke formation, and accelerated catalyst deactivation inn-pentane catalytic cracking.
In our previous work,n-pentane catalytic cracking over HZSM-5 zeolites was systematically investigated. It was addressed that light olefins formation was controllable by tailoring zeolite properties and operating conditions; while the increase of light olefins selectivity would accelerate the deactivation of HZSM-5 zeolites[16-19]. Recently, the unique links between alkenes formation, coke location, and HZSM-5 deactivation inn-pentane catalytic cracking revealed that an increase of alkenes selectivity enhanced the external coke formation on HZSM-5 zeolites, while blocking pore openings and accelerating catalyst deactivation[20]. This work was intended to study the effects of operating conditions on the catalytic performance of HZSM-5 zeolites. Catalytic cracking ofn-pentane was carried out over HZSM-5 zeolites at 650 °C under atmosphere pressure for 100 min on stream.Operating conditions, including the weight hourly space velocity, the reactant partial pressure, and the carrier gas flow rate, were tailored, and the variations ofn-pentane conversion, light olefins production, product distribution,coke deposit, etc., were measured. Several reaction indexes were defined to evaluate the effects of operating conditions on the catalytic performance of HZSM-5 zeolites.
2 Experimental
2.1 Reactant, carrier gas and catalyst
n-Pentane (99.5%) was purchased from the Huadong Reagent Company (Tianjin, China), and used without further purification. High purity nitrogen (N2, 99.999%)was used as carrier gas without further purification.HZSM-5 zeolites with a SiO2/Al2O3ratio of 25 were purchased from the Nankai Catalyst Company (Tianjin,China). HZSM-5 zeolites were calcinated in dry air at 550 °C for 6 h, and then tableted, crushed, and sieved into 30—40 mesh range before use. The physical and chemical properties of HZSM-5 zeolites were detected and presented by the previous work, which confirmed the existence of microporous structure and abundant acid sites[20].
2.2 n-Pentane catalytic cracking
n-Pentane catalytic cracking was conducted in a fixedbed reactor, which was introduced in details by the previous work[16,18,21]. The mixture of HZSM-5 zeolites and SiC was fixed at the middle zone of reactor tube by the quartz wool plugs and copper wire mesh. Prior to the cracking test, the reactor tube and catalyst were pretreated at 150 °C in N2flow for 1 h. Then,n-pentane was introduced and the reaction temperature was raised up to 650 °C, which was maintained for 100 min on stream. The cracked gas was sampled and analyzed by an online gas chromatograph. As shown in Table 1, HZSM-5 zeolites amount,n-pentane feed rate, and N2flow rate were in the range of 100—400 mg, 0.6—1.8 mL/h, and 150—250 mL/min, respectively. Weight hourly space velocity and reactant partial pressure were calculated,with the results summarized in Table 1.
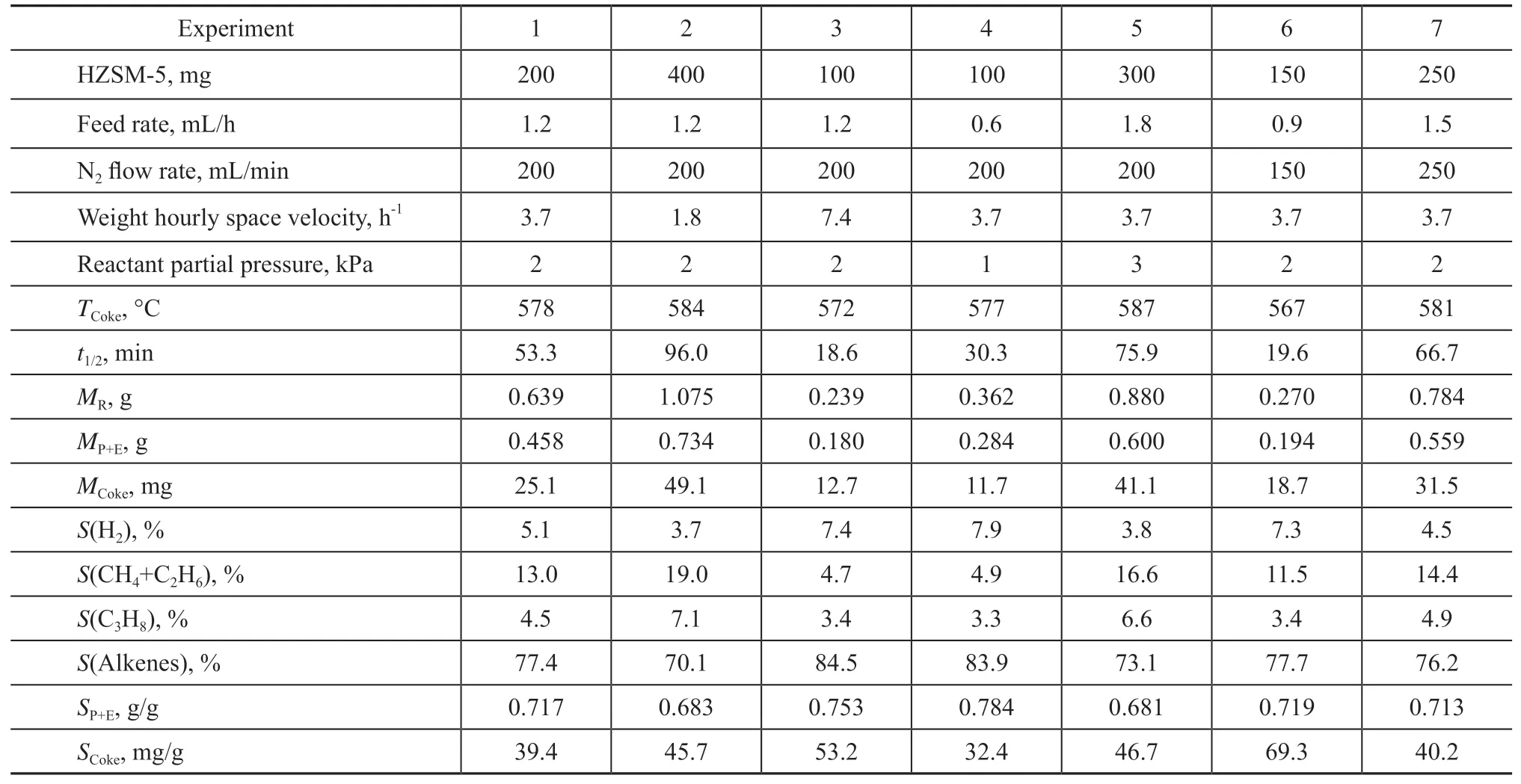
Table 1 Operating conditions and reaction indexes for n-pentane catalytic cracking over HZSM-5 zeolites
2.3 Coke analysis
Temperature programed oxidation (TPO) was conducted to analyze coke deposit on the spent HZSM-5 zeolite samples. As a typical run, the spent HZSM-5 zeolite(ca. 10 mg) was first dried at 100 °C for 0.5 h; then,the temperature was raised up to 800 °C at a jump rate of 10 °C/min in air flow (60 mL/min). The weight loss of the spent HZSM-5 zeolite samples was recorded and presented as a function of temperature, which was denoted as TG curves. DTG curves were obtained by differentiating TG curves against temperature.
2.4 Definition of reaction indexes
As shown in Table 1, eleven reaction indexes were defined and calculated to characterize the catalytic performance of HZSM-5 zeolites inn-pentane cracking:(1) The “TCoke” was defined as coke burning temperature and represented by the central temperature of DTG curve for coke combustion. A higherTCokeindicated more internal coke in HZSM-5 micro pores, and a lowerTCokeindicated more external coke on HZSM-5 external surface[22-23]. (2) The “t1/2” was defined as HZSM-5 halflifetime, which was represented by the TOS value, the corresponding conversion of which was half of the initial conversion at TOS=0 min. It indicated the catalytic stability of HZSM-5 zeolites inn-pentane cracking.(3) The “MR,MP+EandMCoke” were defined asn-pentane consumption, ethylene plus propene production, and coke accumulation for 100 min on stream, respectively.(4) The “S(H2),S(CH4+C2H6),S(C3H8) andS(Alkenes)”were defined as hydrogen selectivity, methane plus ethane selectivity, propane selectivity, and ethylene, propene plus butene selectivity withn-pentane conversion being close to ca. 40%. (5) The “SP+E” and “SCoke” were defined as the average selectivity to ethylene plus propene and the selectivity to coke for 100 min on stream, and represented by the ratio ofMP+EandMCoketoMR, respectively.
3 Results
3.1 Effects of weight hourly space velocity
As show in Figure 1(a), an increase of weight hourly space velocity could hardly affect the initial conversion and yield of ethylene plus propene; while, it would accelerate the deactivation of HZSM-5 zeolites. For example,n-pentane conversion and ethylene plus propene yield at TOS=100 min were 66.8% and 43.0%with a weight hourly space velocity of 1.8 h-1, while it respectively decreased to 1.5% and 1.1% with an increase of weight hourly space velocity to 7.4 h-1. As shown in Figure 1b, the spent HZSM-5 zeolites had a similar weight loss of 11.2%(±0.15%) in TPO test; meanwhile the coke burning temperature decreased from 584 °C to 572 °C with the weight hourly space velocity increasing from 1.8 h-1to 7.4 h-1. The similar weight loss combining the monotonous decrease of burning temperature indicated that an increase of weight hourly space velocity led to more external coke and less internal coke. As shown in Table 1, an increase of weight hourly space velocity from 1.8 h-1to 7.4 h-1decreased the HZSM-5 half-lifetime from 96.0 min to 18.6 min, while then-pentane consumption decreased from 1.075 g to 0.239 g,the ethylene plus propene production reduced from 0.734 g to 0.180 g, and the coke accumulation dropped from 49.1 mg to 12.7 mg. It was deduced that an increase of weight hourly space velocity enhanced the formation of external coke and accelerated the deactivation of HZSM-5 zeolites, which reduced then-pentane consumption,the ethylene plus propene production, and the coke accumulation. This outcome was consistent with the work of Coelho, et al., who found that an increase of weight hourly space velocity from 13.8 h-1to 80 h-1accelerated HZSM-5 deactivation in the transformation of 1-butene[24].
3.2 Effects of n-pentane partial pressure
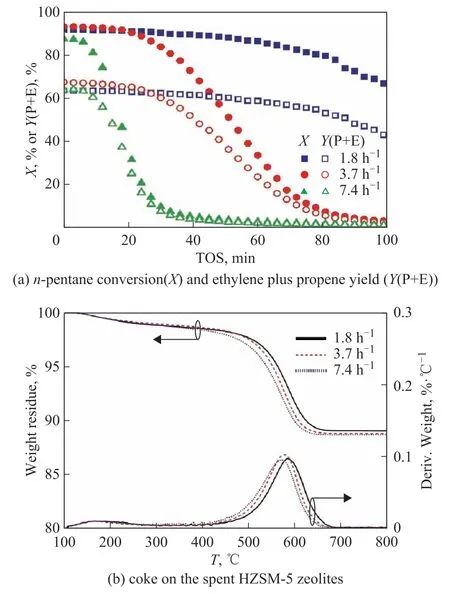
Figure 1 Effects of weight hourly space velocity on n-pentane catalytic cracking
As show in Figure 2(a), an increase of reactant partial pressure could hardly affect the initial conversion and yield of ethylene plus propene; meanwhile, it alleviated the deactivation of HZSM-5 zeolites. For example, then-pentane conversion and ethylene plus propene yield at TOS=100 min were 2.2% and 1.6% with a reactant partial pressure of 1 kPa; while then-pentane conversion and ethylene plus propene yield increased to 21.5%and 13.7%, respectively, with an increase of reactant partial pressure to 3 kPa. As shown in Figure 2b, the spent HZSM-5 zeolites held the similar weight loss of 11.3%(±0.80%) in TPO test; meanwhile the coke burning temperature increased from 577 °C to 587 °C with an increase of reactant partial pressure from 1 kPa to 3 kPa.The similar weight loss along with the monotonous increase of burning temperature indicated that an increase of reactant partial pressure led to less external coke and more internal coke. As show in Table 1, increasing the reactant partial pressure from 1 kPa to 3 kPa increased the HZSM-5 half-lifetime from 30.3 min to 75.9 min,then-pentane consumption from 0.362 g to 0.880 g, the ethylene plus propene production from 0.284 g to 0.600 g,and the coke accumulation from 11.7 mg to 41.1 mg. It was deduced that an increase of reactant partial pressure inhibited the formation of external coke and alleviated the deactivation of HZSM-5 zeolites, which promoted then-pentane consumption, the ethylene plus propene production, and the coke accumulation.
3.3 Effects of N2 flow rate
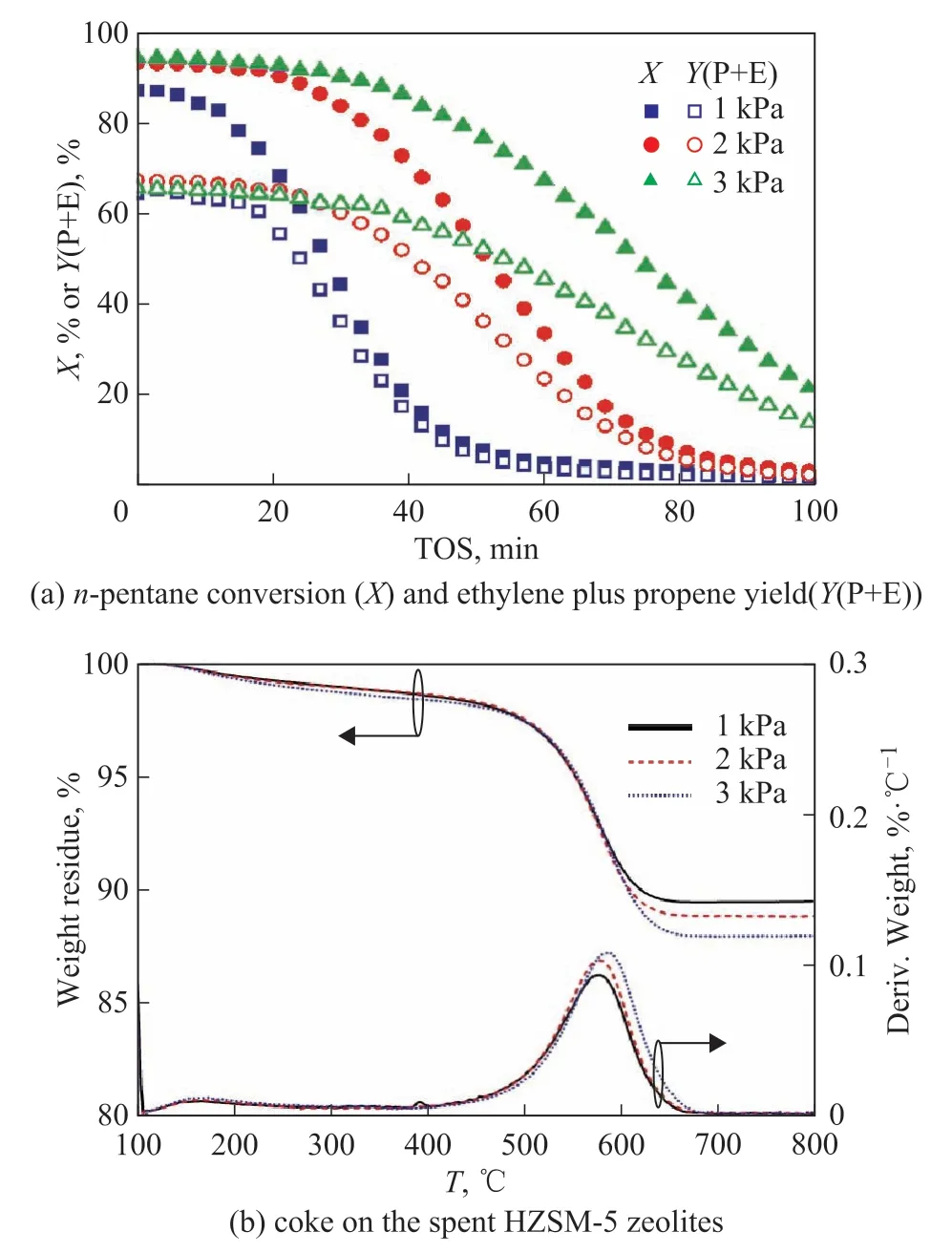
Figure 2 Effects of reactant partial pressure on n-pentanecatalytic cracking
As show in Figure 3a, an increase of N2flow rate could hardly affect the initial conversion and the yield of ethylene plus propene, while it alleviated the deactivation of HZSM-5 zeolites. For example, then-pentane conversion and the ethylene plus propene yield at TOS=100 min reached 2.1% and 1.5%, respectively,with a N2flow rate of 150 mL/min, while then-pentane conversion and the ethylene plus propene yield increased to 9.1% and 6.5%, respectively, with an increase of N2flow rate to 250 mL/min. As shown in Figure 3b, the spent HZSM-5 zeolites held the similar weight loss of 11.2%(±0.06%) in TPO test; meanwhile, the coke burning temperature increased from 567 °C to 581 °C with an increase of N2flow rate from 150 mL/min to 250 mL/min.The similar weight loss along with the monotonous increase of burning temperature indicated that an increase of N2flow rate led to less external coke and more internal coke. As shown in Table 1, increasing the N2flow rate from 150 mL/min to 250 mL/min could increase HZSM-5 half-lifetime from 19.6 min to 66.7 min, then-pentane consumption from 0.270 g to 0.784 g, the ethylene plus propene production from 0.194 g to 0.559 g, and the coke accumulation from 18.7 mg to 31.5 mg. It was deduced that an increase of N2flow rate inhibited the formation of external coke and alleviated the deactivation of HZSM-5 zeolites, which promoted then-pentane consumption,the ethylene plus propene production, and the coke accumulation. Hodoshima, et al.[9]found that the presence of carrier gas helped to alleviate catalyst deactivation,which was consistent with this work.

Figure 3 Effects of N2 flow rate on n-pentane catalytic cracking
4 Discussion
According to the experimental results, a general understanding was achieved about the effects of operating conditions on the catalytic performance of HZSM-5 zeolites inn-pentane cracking, i.e. decreasing the weight hourly space velocity, increasing the reactant partial pressure, and increasing the N2flow rate inhibited the formation of external coke and alleviated the deactivation of HZSM-5 zeolites, which promotedn-pentane consumption, ethylene plus propene production, and coke accumulation. As shown in Figure 4,n-pentane consumption, ethylene plus propene production, and coke accumulation linearly increased with HZSM-5 half-lifetime, indicating that reactant transformation,light olefins production, and coke accumulation were mainly determined by the catalytic stability of HZSM-5 zeolites. However, the catalytic stability of HZSM-5 zeolites was closely related to the coke formation and its location. Operating conditions, product distribution, coke formation, and half-lifetime of HZSM-5 zeolites were all connected by the reaction network[16,18-20].
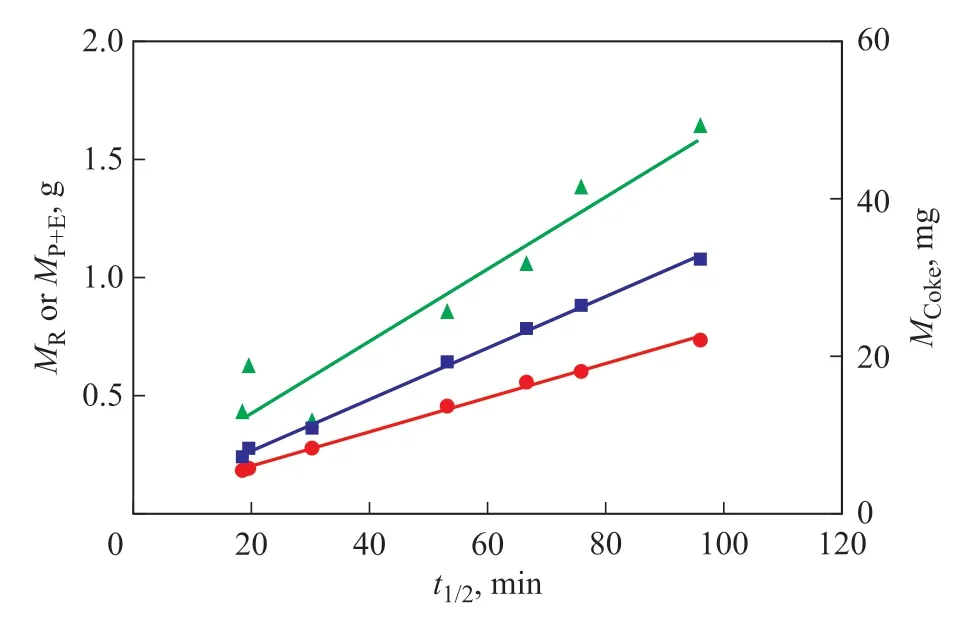
Figure 4 MR, MP+E and MCoke as a function of HZSM-5 half-lifetime (t1/2) in n-pentane catalytic cracking
According to the literature reports[8,18,25-30], a possible reaction network was proposed to characterize the product distribution inn-pentane catalytic cracking over HZSM-5 zeolites. As shown in Figure 5, the attack of B acid sites onn-pentane led to the protonation of C-C/C-H bonds, and generated carbonium ions that could be rapidly degraded into carbenium ions as well as hydrogen,methane or ethane (monomolecular protolytic cracking).The carbenium ions can react withn-pentane generating propane (hydride transfer reactions), restore B acid sites by generating propene and butene (deprotonation), or can decompose into smaller carbenium ions coupled with ethylene formation (β-scission). At the same time, the generated alkenes can react with B acid sites generating carbenium ions (protonation), or react with carbenium ions generating complex hydrocarbons and even coke deposits (side reactions). The reaction pathways were connected with one or several specific products duringn-pentane catalytic cracking over HZSM-5 zeolites[16].
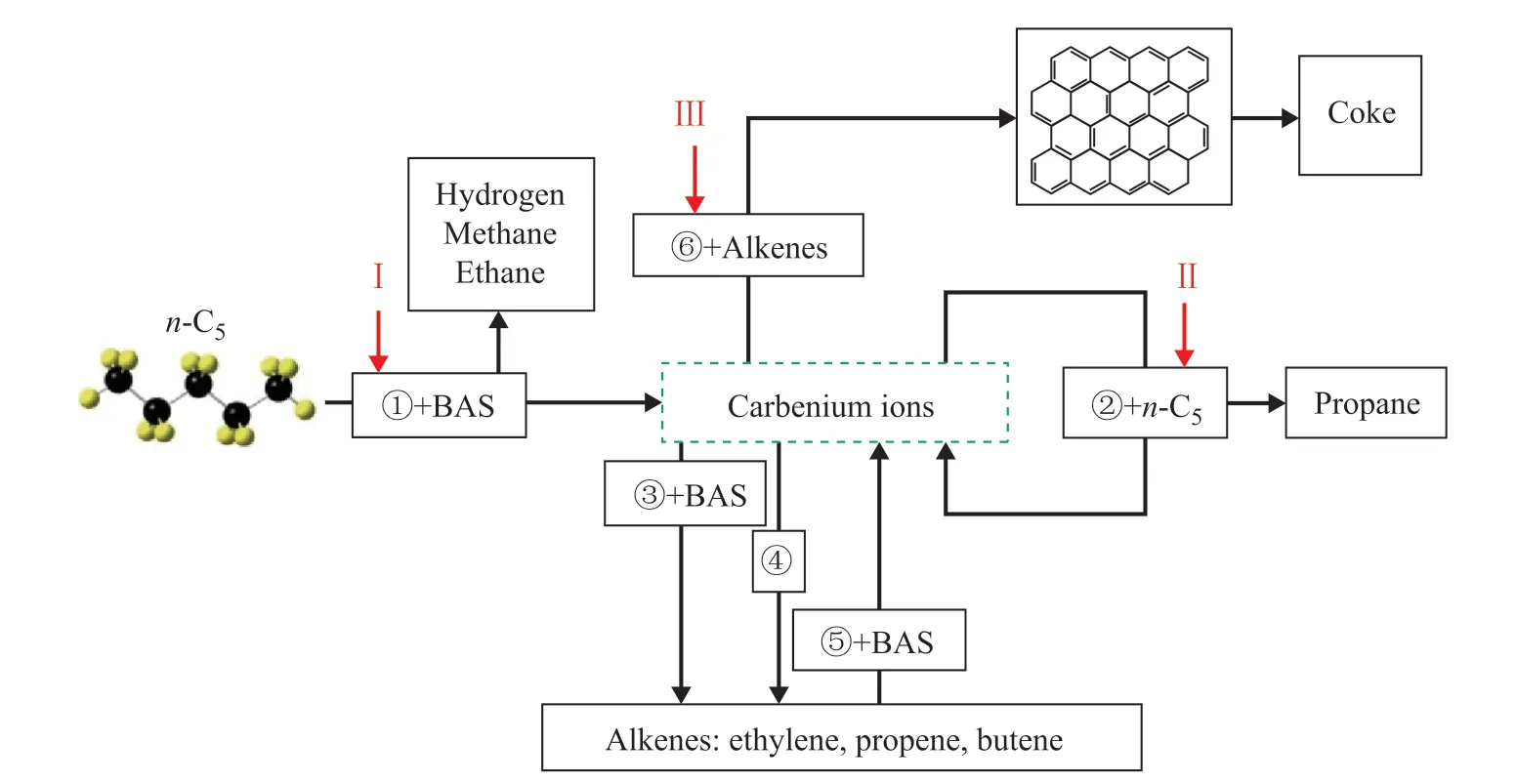
Figure 5 Possible reaction network for n-pentane catalytic cracking over HZSM-5 zeolites
Based on the features of reaction network, the hydrogen selectivity and the methane plus ethane selectivity were employed to depict the C-H/C-C bonds breaking in monomolecular protolytic cracking; the propane selectivity was employed to depict the hydride transfer reactions; the ethylene, propene plus butene selectivity was employed to depict the alkenes formation. As shown in Figure 6, decreasing the weight hourly space velocity,increasing the reactant partial pressure, and increasing the N2flow rate could promote the methane plus ethane selectivity and the propane selectivity while reducing the hydrogen selectivity. It was deduced that decreasing the weight hourly space velocity, increasing the reactant partial pressure, and increasing the N2flow rate could inhibit the C-H bonds breaking while enhancing the C-C bonds breaking and the hydride transfer reactions,which contributed negatively to alkenes formation along with improved catalytic stability of HZSM-5 zeolites.Compared with the case for decreasing the weight hourly space velocity and increasing the reactant partial pressure,increasing the N2flow rate would sacrifice less alkenes selectivity, which might be attributed to the unique roles of carrier gas in the reaction network.
As shown in Figure 7, the average selectivity to propene plus ethylene and the average coke selectivity were presented as a function of HZSM-5 half-lifetime. It was observed that the improvement of catalytic stability achieved by increasing N2flow rate was accompanied with the stable selectivity to ethylene plus propene and the decreased selectivity to coke, while the improvement of catalytic stability achieved by decreasing the weight hourly space velocity and increasing the reactant partial pressure was accompanied with the decreased selectivity to ethylene plus propene coupled with the decreased or increased selectivity to coke. Considering the unique effects of carrier gas, it was proposed that the carrier gas was able to regulate the diffusion process and the secondary reactions related to alkenes. Increasing the carrier gas flow rate could promote the diffusion of products and inhibit the secondary reactions of alkenes. It hindered the routes from alkenes to coke formation and protected alkenes from consumption.This was the reason why increasing the N2flow rate could lead to a satisfactory balance between the ethylene plus propene selectivity and the catalytic stability. Thus,tailoring carrier gas can be a candidate method to improve the catalytic performance of HZSM-5 zeolites inn-pentane cracking for producing light olefins.
5 Conclusions
Briefly, effects of operating conditions on the catalytic performance of HZSM-5 zeolites inn-pentane cracking were studied in this work. It was found that decreasing the weight hourly space velocity, increasing the reactant partial pressure, and increasing the N2flow rate could inhibit the C-H bond breaking and enhance the C-C bond breaking and hydride transfer reactions to reduce alkenes selectivity, which suppressed the formation of external coke and alleviated the deactivation of HZSM-5 zeolites.It was deduced that the catalytic stability of HZSM-5 zeolites was improved at the cost of alkenes selectivity.However, the improvement of catalytic stability achieved by increasing the N2flow rate sacrificed less alkenes selectivity as compared to the case for decreasing the weight hourly space velocity and increasing the reactant partial pressure. Considering the unique effects of carrier gas, it was proposed that increasing the carrier gas flow rate could enhance the diffusion process and inhibit the secondary reactions of alkenes. It hindered the routes from alkenes to coke formation and protected alkenes from consumption. Tailoring carrier gas can be a candidate method to improve the catalytic performance of HZSM-5 zeolites inn-pentane cracking for producing light olefins.Acknowledgments: The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21908010), the Education Department of Jilin Province (Grant No. JJKH20191314KJ), and the Changchun University of Technology.
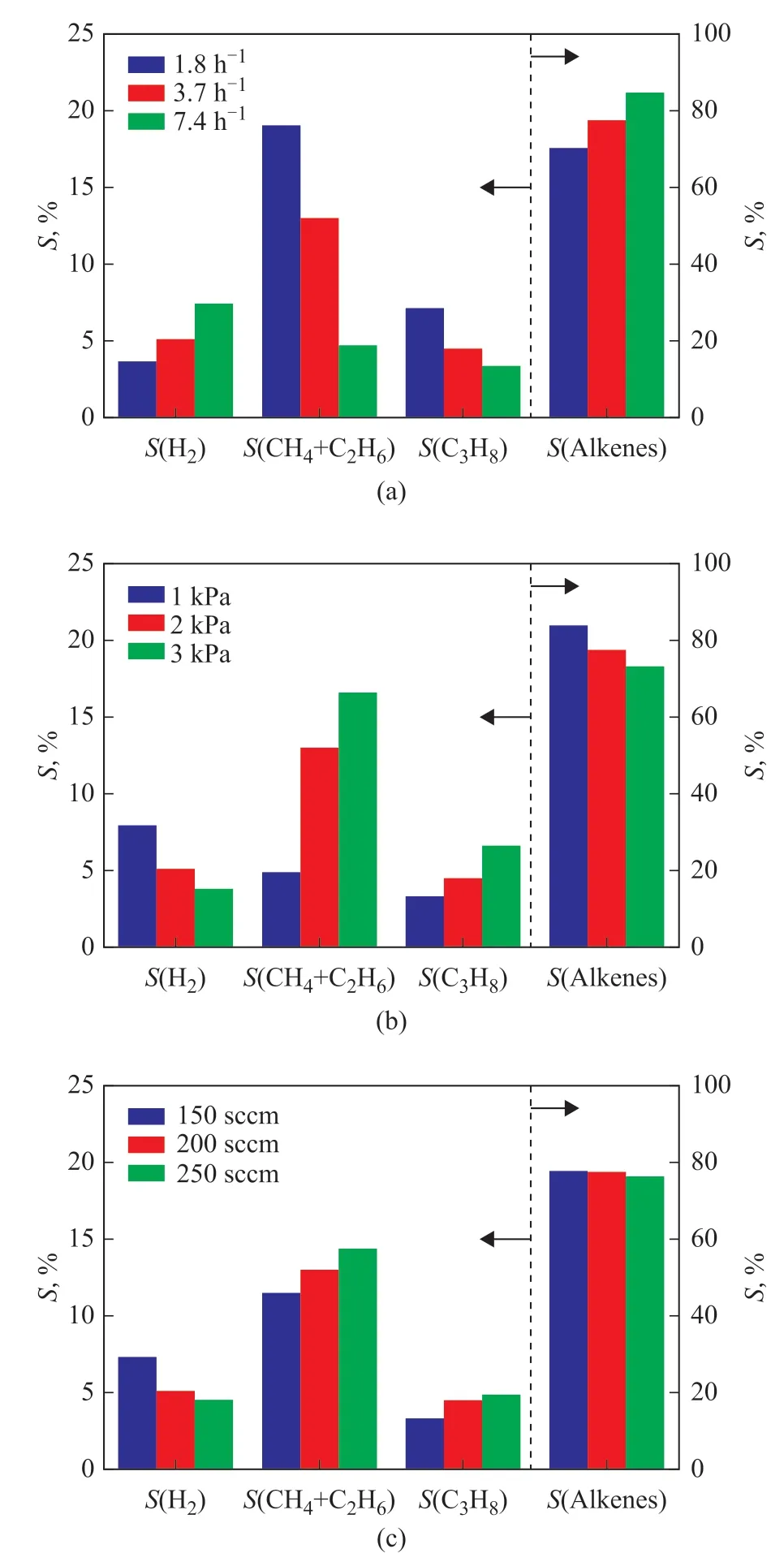
Figure 6 Effects of (a) weight hourly space velocity, (b)reactant partial pressure and (c) N2 flow rate on S(H2),S(CH4+C2H6), S(C3H8) and S(Alkenes) in n-pentane catalytic cracking over HZSM-5 zeolites
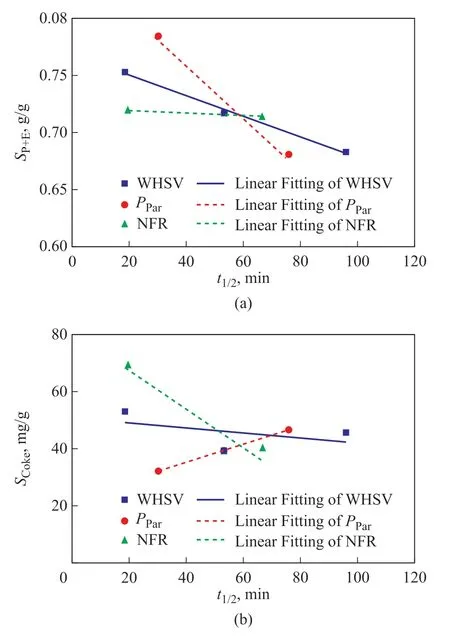
Figure 7 Average selectivity (a) to propene plus ethylene(SP+E) and (b) to coke (SCoke) as a function of HZSM-5 halflifetime in n-pentane catalytic cracking(NFR: N2 flow rate; WHSV: weight hourly space velocity;Ppar: reactant partial pressure)
- 中國煉油與石油化工的其它文章
- Preparation and Rheological Properties of Vacuum Lubricating Grease
- Effects of Different Plant Hormones for Microbial Degradation of PASHs and Diesel under Aerobic Conditions
- Nitrogen Removal Performance of Denitrifying Ammonium Oxidation System in Treating Sulfamethoxazole-laden Secondary Wastewater Effluent
- Removal of Basic Nitrogen Compounds from Diesel Fraction with NMP-0.5ZnCl2 Coordinated Ionic Liquid
- Flow Characteristics of Crude Oil with High Water Fraction during Non-heating Gathering and Transportation
- Comparison and Analysis of Toluene Adsorption Properties of ZSM-5 Molecular Sieve Treated by Different Modification Methods: Adsorption Kinetic and Mechanism Studies

