Comparison and Analysis of Toluene Adsorption Properties of ZSM-5 Molecular Sieve Treated by Different Modification Methods: Adsorption Kinetic and Mechanism Studies
Zhang Shuai; Li Meng; Li Wei; Li Yuwei; Liu Fang,;Xue Ming; Wang Yongqiang,; Zhao Chaocheng
(1. College of Chemical Engineering, China University of Petroleum, Qingdao 266580;2. State Key Laboratory of Petroleum Pollution Control, Beijing 102206)
Abstract: MFI molecular sieve with high specific surface area, adjustable pore size and low production cost have been recognized as an effective adsorbent for VOCs removal. In this paper, NaOH solution was used to etch ZSM-5 to increase specific surface area and micro-mesoporous content. Graphene oxide (GO) was loaded on ZSM-5 by ultrasonic-assisted heating immersion method, and large π-bond structure and oxygen-containing functional groups were added to ZSM-5 for improving the composite adsorption performance. In addition, the properties of OH-ZSM-5 and ZSM-5@GO composites for toluene adsorption under different factors were also studied. It was positively correlated with initial concentration and adsorbent mass, but was negatively correlated with temperature. Meanwhile, the results showed that the saturated adsorption capacity of OH-ZSM-5 (107.3 mg/g) was 1.34 times higher than that of ZG-15% (80.2 mg/g). The pseudo-first-order and pseudo-second-order kinetic model can well describe the adsorption behavior of toluene on the OH-ZSM-5 and ZG-15%, respectively. The adsorption mechanism of OH-ZSM-5 was mainly pore-filled adsorption. However, the adsorption mechanisms of ZSM-5@GO composite were pore-filled adsorption, π-π interaction, and H-bond interaction. This study will help to design a new strategy for enhancing the performance of traditional adsorbent ZSM-5 in VOCs removal.
Key words: OH-ZSM-5; ZSM-5@GO; volatile organic compounds (VOCs); adsorption properties of toluene; mechanism
1 Introduction
Volatile organic compounds (VOCs), as a kind of harmful and stable industrial pollution sources, seriously affect the atmospheric environment and human health[1]. As one of the most representative VOCs, benzene series is not only an important raw material for fuel and synthetic solvent, but also an indispensable part of rubber industry[2]. Toluene usually exists in the atmospheric environment in the form of gaseous state. It not only can damage the body’s nervous system, irritate the skin and mucous membranes, but it can also cause congenital deformities in fetuses[3]. Therefore, effective treatment of VOCs is of great significance to protect the environment and human health. At present, many technologies have been used, such as adsorption[4], catalytic combustion[5],biological method[6], low temperature condensation,and photocatalysis technology[7]. Among the above technologies, the adsorption method is widely used due to the advantages of simple operation, easy recycling of materials, high efficiency, and low cost.
The core of the adsorption method is the selection of adsorbent[8]. At present, porous carbon, ordered mesoporous silicate[9], graphene[10], and metal organic framework materials (MOF)[4]have been commonly used as the adsorption materials of VOCs removal. However,some disadvantages of adsorbents have limited their wide application in the field of VOCs adsorption, such as the low adsorption and recycling efficiency of activated carbon in humid environment[11], the tedious preparation process of graphene, and the poor water stability of MOFs. ZSM-5 zeolite, featuring high hydrothermal stability, good hydrophobicity[12], high selectivity, unique pore structure, and low production cost, is widely used in the field of VOCs removal[13-15].
At present, the reprocessing methods of ZSM-5 zeolite mainly include acid-base modification, ion-exchange method, impregnation, silanization treatment, and preparation of composite materials. Among them, the measurement of mesoporous content and hydrophobicity of the adsorption material is required by acid-base treatment and silanization treatment, respectively[16]. The impregnation and preparation of composite materials can provide ZSM-5 zeolite with new properties. Zhang group[17]prepared a Zn-ZSM-5 material through an alkaline treatment and co-impregnation method. The stability of the modified material was enhanced by the mesopores generated by the alkaline treatment, and the increase of Zn content increased the BTEX yield of the adsorption material. Li group[18]prepared the Cu-BTC@ZSM-5 composite via the ethanol activationassisted hydrothermal method, and the result showed that the capacity for adsorption of toluene was 158.6 mg/g in a humid environment. As a new nanoporous material, graphene oxide (GO) has the characteristics of high specific surface area, abundant negative oxygencontaining functional groups, and rich pore structure.Its adsorption performance can be further enhanced by compounding with other materials. Li group[19]found that GO entered the Si-ZSM-5 crystals to form a layered mesoporous structure. Du group[20]synthesized the graded GO-Beta composites via the hydrothermal method, and found that the layered structure, type, and content of GO were beneficial to the formation of mesopores and the adjustment of mesoporous rate. Kim group[21]synthesized the composite of GO with octahedral zeolite via a template free method, and the result showed the removal efficiency of MB was as high as 99%.
In this paper, the ZSM-5 zeolite was selected as the matrix material, and the adsorption materials of VOCs were prepared by NaOH solution immersion etching method and ultrasonic-assisted heating immersion method,respectively. In the dynamic adsorption experiment, the properties of ZSM-5, GO, OH-ZSM-5 and ZSM-5@GO for adsorption of toluene were compared. The influence of ZSM-5 content, toluene initial concentration, temperature,adsorbent quality on the adsorption capacity of the two materials were further studied. Finally, the adsorption kinetics and mechanisms of OH-ZSM-5 and ZSM-5@GO composites were analyzed.
2 Experimental
2.1 Materials
Flake graphite was purchased from the Qingdao Risheng Graphite Co., Ltd.; H-ZSM-5 zeolite was procured from the Nankai University Catalyst Factory; NaNO3, H2O2,NaOH, toluene, carbon disulfide (CS2), CH3CH2OH, and polyethylene glycol 400 (PEG400) were bought from the Sinopharm Chemical Reagent Co., Ltd.; HNO3, H2SO4, and HCl were procured from the Xilong Chemical Co., Ltd.;NH4NO3was bought from the Yantai Shuangshuang Chemical Co., Ltd.; KMnO4was bought from the Yantai Sanhe Chemical Reagent Co., Ltd.; deionized water was provided by the laboratory. All reagents are of analytical grade.
2.2 Synthesis of GO
GO was prepared by modified Hummer’s oxidation method[4,22]. Firstly, 2 g of graphite powder and 1 g of NaNO3were put into a 1000-mL beaker, 100 mL of H2SO4were added slowly and stirred for about 30 min at 4 °C. Next, 10 g of KMnO4were added evenly and slowly into the above beaker and stirred consecutively for 2 h.Then, the above beaker with the mixture was moved into a thermostatic water bath at 35 °C followed by continued stirring for 1.5 h. Afterwards, 100 mL of H2O (35 °C)were added continuously and slowly to the above 1000-mL beaker, which was then placed into a high-temperature water bath operated at 98 °C under stirring for 3―5 min.After that, 500 mL of H2O (50 °C) and 30 mL of H2O2(30%) were added to remove the residual KMnO4. The above solution was stored overnight and the supernatant was poured out, then the substrate was washed with 5%HCl and H2O for 3 times, respectively. Lastly, the reactant was freeze-dried for 72 h to obtain GO.
2.3 Synthesis of OH-ZSM-5
ZSM-5 was calcinated in muffle furnace at 500 °C for 3 h to remove the template; ZSM-5 was put into a 0.2 mol/L NaOH solution for ultrasonic treatment for 10 min, and then was treated in a constant temperature water bath at 50 °C for 30 min, centrifuged and washed to neutral reaction. The product was dried at 80 °C for 12 h and ion-exchanged in NH4NO3solution, the mixture was centrifugally washed at 4000 r/min for 5 min. Finally,it was calcined in a muffle furnace at 500 °C for 2 h to obtain the alkali-treated ZSM-5 (OH-ZSM-5).
2.4 Synthesis of ZSM-5@GO
Firstly, an appropriate amount of GO prepared by the above-mentioned procedure and some deionized water were placed in a 100-mL beaker and ultrasonically dispersed for 1 h to obtain a suspension containing 5 mg/mL of GO. After that, the ZSM-5 powder was added to the GO suspension. The suspension was mixed and ultrasonically treated for 0.5 h, and was then stirred in a water bath operating at 80 °C until water was completely evaporated. Lastly, the product was ground into powder.The GO mass content in the composite materials was 5%, 10%, 15%, 20%, 25%, and 40%, respectively. The synthesized composites were referred to as ZG-X%depending on the GO content.
2.5 Characterization
Transmission electron microscope (TEM) images were taken by a JEOL JEM-2100UHR electron microscope(Japan Electronics Co., Ltd.), which was used to observe the morphology and crystal structure of the material. The surface groups were obtained by a VERTEX70v in FTIR spectrometer (Bruker, Germany). The characterization of the crystal structure of the composites was observed by X-ray diffraction (XRD) (Panaco Empyrean Sharp,Netherlands). The N2adsorption-desorption isotherms were measured on an ASAP2020-M surface area analyzer(Micromeritics, USA). The specific surface area, pore size distribution and pore volume were calculated by the Brunauer-Emmett-Teller (BET) method, the Barrett-Joyneer-Halenda (BJH) method, and the t-plot method,respectively.
2.6 Dynamic adsorption experiment
Figure 1 shows the structure of gaseous toluene dynamic adsorption system. Gaseous toluene was generated by air bubbling method, and the total gas flow rate was maintained at 180 mL/min, which was controlled by a flow meter and kept stable. The toluene generator and air reactor were controlled by the gas flowmeters,respectively. The sampling tube was connected to the sampling point and the outlet to collect the initial and adsorbed gas samples, respectively, by an 100-mL glass syringer. PEG400 was used to absorb the residual toluene gas.
The air tightness and stability of the device should be ensured before starting the experiment. The toluene concentration was detected by CS2solvent desorption and gas chromatography method. The specific steps can refer to the previously reported literature[4]. The adsorption breakthrough time and the saturation time were assumed to be the time required to reachC/C0=0.05 and 0.95,respectively.
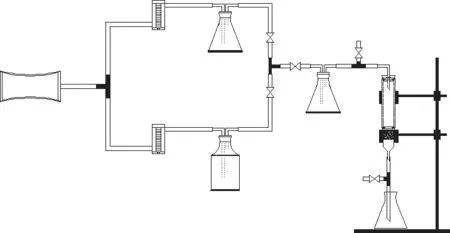
Figure 1 The Schematic of the toluene dynamic adsorption system
The effects of GO content, initial toluene concentration,temperature, and adsorbent mass on the adsorption capacity of different adsorbents were investigated,respectively. Meanwhile, the adsorption properties of the two materials under the same conditions were compared. The GO content was adjusted to 5%, 10%,15%, 20%, 25%, and 40% by weight. The initial toluene concentrations were 900―2 200 mg/m3. The adsorbent mass was 0.2 g, 0.3 g, and 0.4 g, respectively. The experimental temperature was controlled at 25 °C, 35 °C,and 45 °C, respectively.
The amount of toluene adsorption was calculated by the following Eq.(1).

wherem0andmare the mass of initial and saturated adsorption column, respectively.
3 Characterization Analysis
Figure 2 shows the TEM images of different materials. As shown in Figure 2a & 2b, the ZSM-5 zeolite had a smooth surface and prismatic structure, indicating that ZSM-5 had a good crystallinity. However, it can be seen from Figure 2c & 2d that after NaOH treatment, the surface of OH-ZSM-5 became rough and cracks appeared at the edge, which might provide more micro-mesopores[23]. As presented in Figure 2e & 2f, the appearance of wrinkles in the middle of GO could be attributed to insufficient oxidation or overlapping of GO layers. However, the thinner edge of GO had no overlap, indicating that the edge was fully oxidized. In Figure 2g & 2h, the fold-like structure was wrapped on ZSM-5, indicating that GO was successfully supported on the surface of ZSM-5 crystal.As shown in Figure 3a, all the samples presented the adsorption peaks at 3 443 cm-1and 1 636 cm-1, which were originated from the -OH peak of adsorbed water and the deformation vibration peak of H2O, respectively,indicating that the materials still contained a trace of internal water[24]. For GO, the adsorption peak at 1 729 cm-1was ascribed to the C=O stretching vibration peak of -COOH, and the peaks at 1 606 cm-1and 1053 cm-1were attributed to C-O-C and C-O vibration adsorption peak, respectively. For ZSM-5, the peak at 1 227 cm-1was the asymmetric stretching vibration peaks of Si-O tetrahedron[25], and the vibration frequency changed with the change of O-T-O (T=Si, Al) bond length. The stretching vibration peaks at 1 089 cm-1and 778 cm-1were attributed to the Si-O-Si asymmetric vibration peaks[26]. The peaks at 546 cm-1and 448 cm-1corresponded to the characteristic double-ring vibration of the skeleton and T-O (T = Si, Al) bending vibration absorption peak[27]. The spectra of OH-ZSM-5 and ZG-15% were basically similar to that of ZSM-5. For OHZSM-5, it still exhibited the characteristic peak shape of ZSM-5 through NaOH treatment. However, the peak strength and width were changed, indicating that a part of Si species was successfully removed[28]. In addition, a new vibrational peak at 1 733 cm-1of ZG-15% corresponded to the C=O stretching vibration peak of -COOH[29-30],indicating that the composite was successfully prepared.

Figure 2 SEM and TEM images of ZSM-5(a, b), OH-ZSM-5(c, d), GO (e, f) and ZG-15%(g, h)
The peak of GO at 2θ=11.3° was attributed to the diffraction peak on the crystal surface of graphite(001). The crystal structure of GO was formed after the destruction of graphite structure by strong oxidation,and the graphite layer spacing was expanded by the intercalation effect to form folds[31]. For ZSM-5, the diffraction peaks of ZSM-5 were detected at 2θ=7.81°,8.82°, 23.03°, 23.78° and 24.31°, corresponding to (101),(200), (501), (151), and (303), respectively, indicating that ZSM-5 has a stable MFI crystal structure[19]. The spectra of OH-ZSM-5 and ZG-15% were basically similar to that of ZSM-5. However, the peak strength and width of characteristic peaks of ZSM-5 was changed by NaOH treatment and the presence of GO, and the materials still retained the MFI crystal structure[32-33].
N2adsorption isotherm and pore size distribution curves of materials are presented in Figure 4. All materials exhibited a hysteresis loop. As classified by IUPAC,ZSM-5 and OH-ZSM-5 presented the type-I adsorption and the type-H4 hysteresis loop. However, GO and ZG-15% presented the type-IV adsorptions and the type-H4 hysteresis loop, respectively[34]. The hysteresis loop was caused by the capillary condensation, which led to the non-coincidence of isotherms in absorption-desorption process in the medium-high pressure region. The type-I and type-IV adsorption patterns indicated the existence of micropores and micro-mesoporous structure, respectively.The hysteresis loop of ZG-15% was significantly bigger than that of ZSM-5, indicating that the existence of GO could increase the mesoporous content[35]. Table 1 depicts the specific surface area, average pore volume and pore size of materials. It was calculated that OH-ZSM-5 had higher specific surface area (341.68 m2/g) and larger average pore size (5.9 nm) than ZSM-5. However, ZG-15% had lower specific surface area (294.52 m2/g) and smaller average pore size (3.83 nm), showing that NaOH treatment could better improve the performance of ZSM-5.
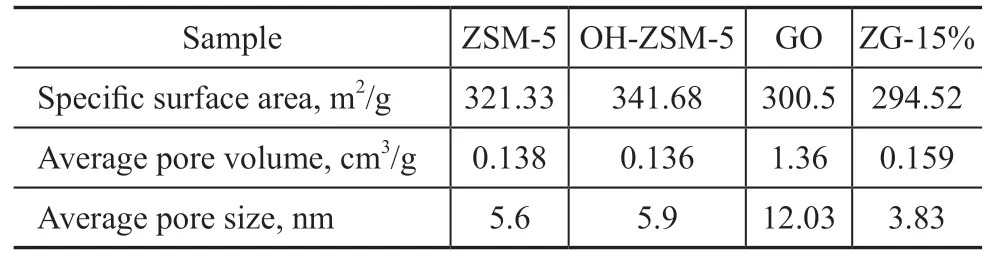
Table 1 BET data of materials
4 Dynamic Adsorption
4.1 Effect of GO contents on toluene adsorption performance
Figure 5 shows the toluene adsorption breakthrough curves on different ZG-X% (X= 5, 10, 15, 20, 25, and 40). Table 2 lists the related parameters. With the increase of GO content, the adsorption curve first shifted to the right and then shifted to the left, and the adsorption rate was accelerated, while the adsorption time was shortened,and finally the adsorption gradually reached saturation.In addition, the penetration time, saturation time and saturated adsorption amount all increased first and then decreased. In Table 2, the toluene adsorption capacity of ZG-15% composite was 80.2 mg/g, and compared with the previously studied ZSM-5 monomer (in Table 2),the adsorption capacity increased by 36.7%, while the adsorption capacity of ZG-40% composite was only 38.6 mg/g. It can be attributed that the abundant π-π bonds and negatively oxygen-containing functional groups of GO can provide more active sites for the adsorption of toluene. However, the excessive content of GO could lead to the blockage of the pores, and the decrease of the adsorption properties.
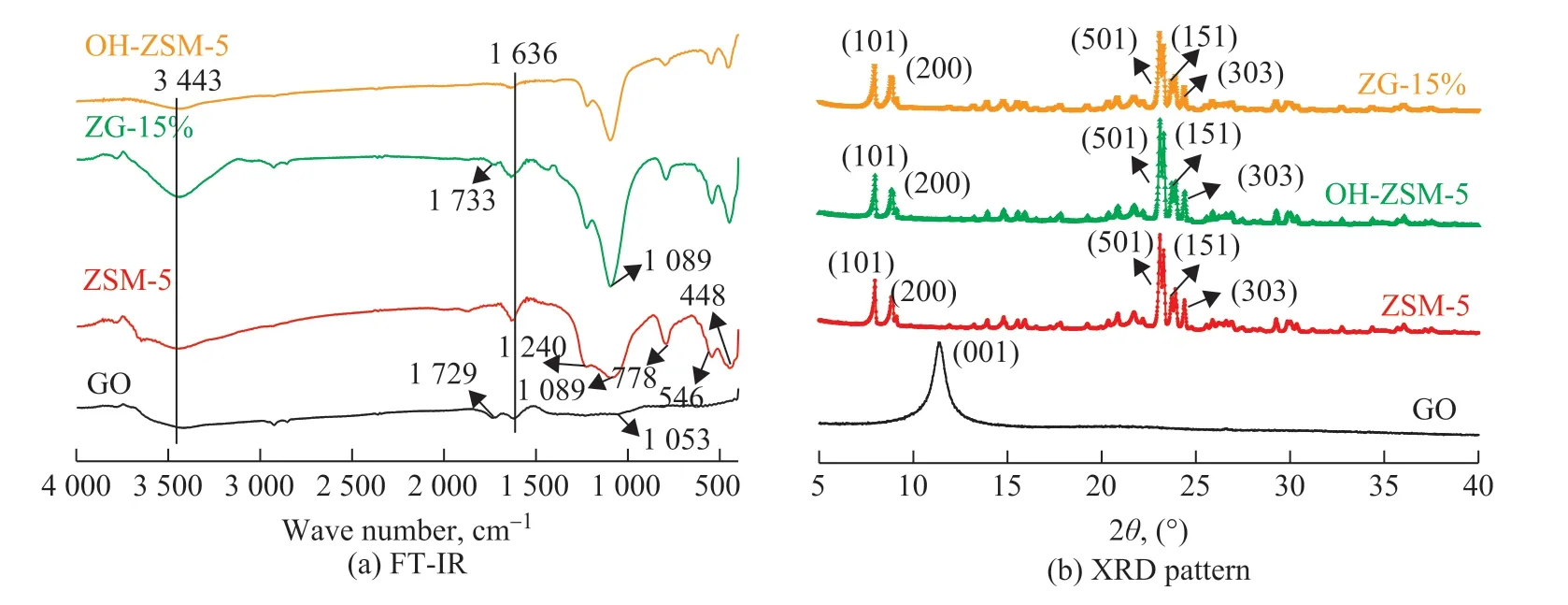
Figure 3 FT-IR (a) and XRD (b) patterns of GO, ZSM-5, ZG-15%, OH-ZSM-5
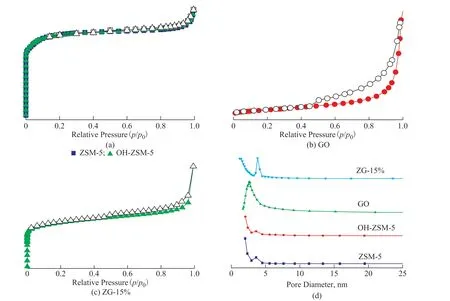
Figure 4 N2 adsorption isotherm(a―c) and pore size distribution(d) of materials
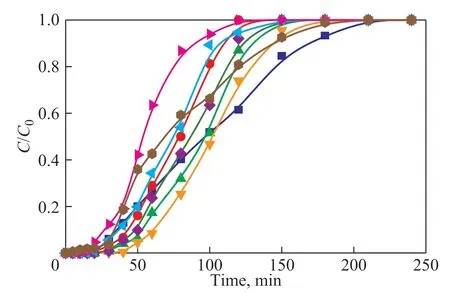
Figure 5 The adsorption curves of different GO contents(The temperature, adsorbent mass, and initial toluene concentration were 25 °C, 0.3 g, and 1 300 mg/m3, respectively)
4.2 Effect of initial toluene concentration on adsorption capacity of OH-ZSM-5 and ZG-15%
Figure 6 shows the toluene adsorption breakthrough curves for different initial toluene concentration(900―2 200 mg/m3). In Figure 6, the tendency oftoluene adsorption curves was similar, with the increase of toluene concentration, the curves became steeper and moved to the left, which indicated that the adsorption rate was accelerated, and the adsorbent material could reach saturation in a short time. The penetration time and saturation time of the composites were negatively correlated with the initial concentration, while the saturated adsorption amount of toluene was positively correlated with the initial toluene concentration. It can be attributed to the fact showing that the higher toluene concentration increased the frequency of collisions between the toluene molecule and the micro mesoporous structure, and the functional groups contained on the composite surface. In addition, under the same conditions, the adsorption time and saturated adsorption capacity of OH-ZSM-5 were higher than that of ZG-15%, indicating that OH-ZSM-5 had better adsorption performance.
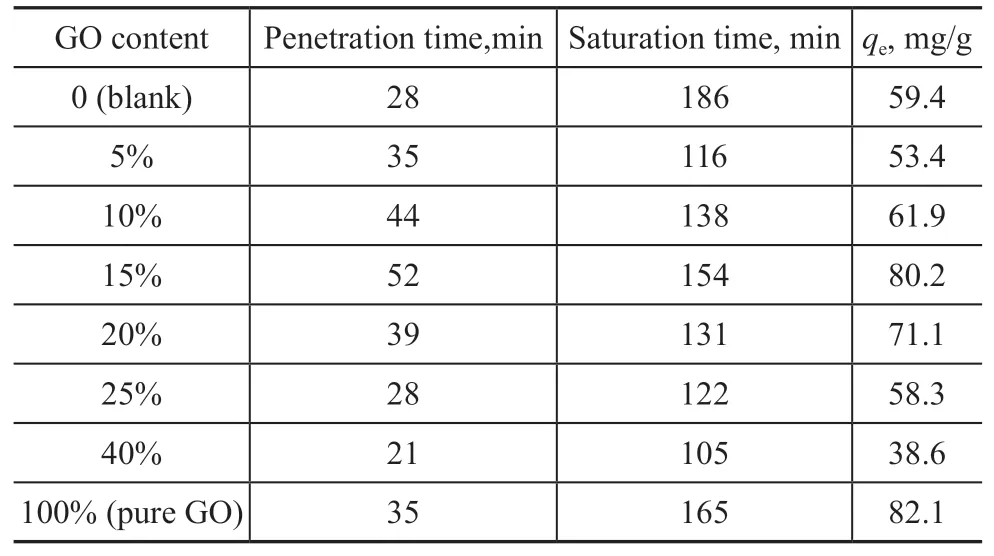
Table 2 Related parameters of toluene under different GO contents
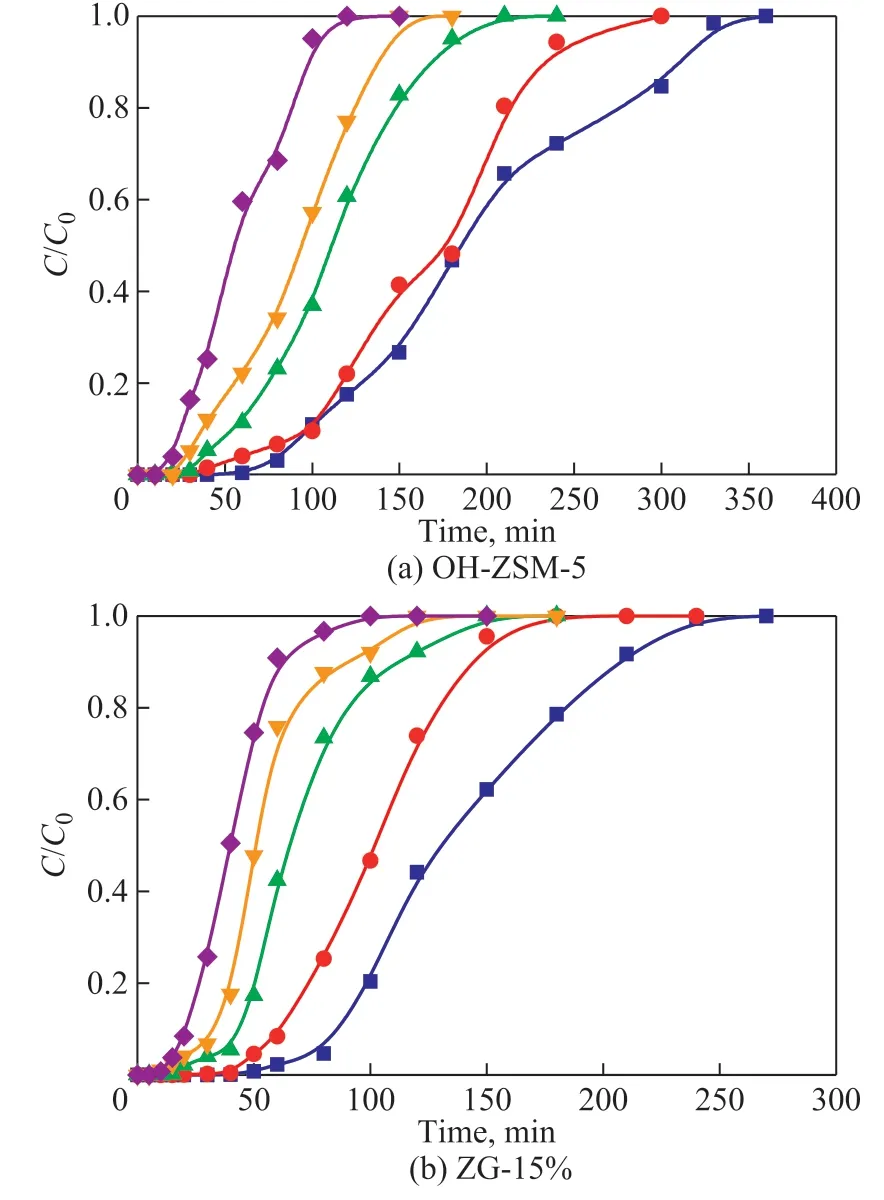
Figure 6 The adsorption curves of different initial toluene concentrations(GO content, temperature, and adsorbent mass were 15%,25 °C, and 0.3 g, respectively)
4.3 Effect of adsorbent mass on adsorption capacity of OH-ZSM-5 and ZG-15%
Figure 7 shows the toluene adsorption breakthrough curves on different adsorbent mass (0.2 g, 0.3 g, 0.4 g).With the increase of adsorbent mass, the curves moved to right. The breakthrough time, saturation time, and saturated adsorption capacity were positively correlated with the adsorbent mass, indicating that the adsorbent mass mainly changed the breakthrough time of toluene,increased the contact time of toluene molecule and adsorbent, and thus increased the adsorption performance of composites.

Figure 7 The adsorption curves of different adsorbent mass(GO content, temperature, and initial toluene concentration were 15%, 25 °C, and 1 300 mg/m3, respectively)
4.4 Effect of temperature on adsorption capacity of OH-ZSM-5 and ZG-15%
Figure 8 shows the toluene adsorption breakthrough curves at different temperatures (25 °C, 35 °C, 45 °C).With the increase of temperature, the curves moved to left. The breakthrough time, saturation time, and saturated adsorption capacity were negatively correlated with temperature. At 25 °C and 45 °C, the maximum and minimum adsorption capacity of OH-ZSM-5 was 107.3 and 54.2 mg/g, respectively. However, under the same conditions, the adsorption performance of ZG-15% was lower than OH-ZSM-5, since ZG-15% reached its saturated adsorption within 2 h at 45 °C. It can be attributed to the elevated temperature, which intensified the Brownian motion of the toluene molecules.
4.5 Adsorption kinetics of toluene on OH-ZSM-5 and ZG-15%
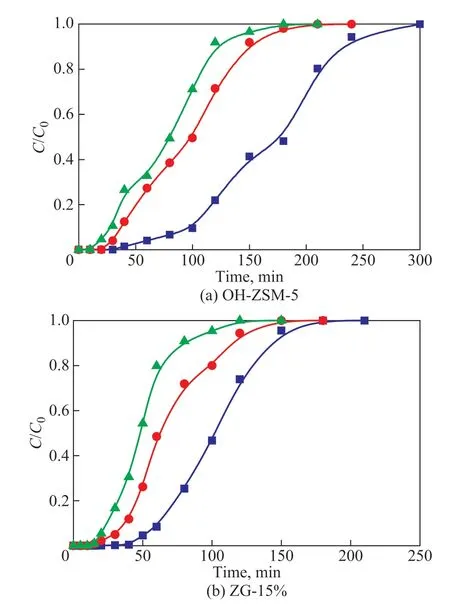
Figure 8 The adsorption curves of different temperature(GO content, adsorbent mass, and initial toluene concentrationwere 15%, 0.3 g, and 1 300 mg/m3, respectively )
The pseudo-first-order (Eq. (2) and (3)) and pseudosecond-order kinetics (Eq. (4) and (5)) were used to describe physical adsorption, physical adsorption and complex chemical adsorption respectively. The diffusion mechanism of toluene in the composites was further studied by using the intraparticle diffusion model Eq. (6).The equations are as follows[4,36-37]:
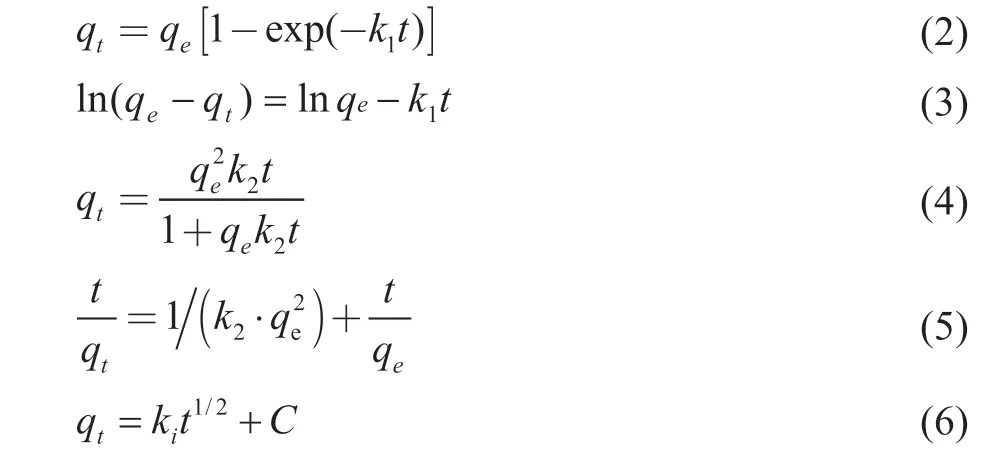
whereqeandqtare the toluene adsorption capacity on composite at the time of adsorption equilibrium andt,respectively (mg/g);k1(min-1) andk2(g/(mg·min)) are the pseudo-first-order and pseudo-second-order kinetics model rate constants, respectively;kiis the rate constant of every stage (mg/(g·min-1/2));Ciis the intercept of section (mg/g).
Figure 9 shows the adsorption kinetics model of toluene adsorption on OH-ZSM-5 and ZG-15%. Table 3 lists the relevant adsorption parameters. As shown in Table 3, for OH-ZSM-5, theseR2of the pseudo-first-order kinetics model were closer to 1 and higher than that of pseudosecond-order kinetics model, indicating that physical adsorption can be used to describe the process. However,for ZG-15%, theseR2of the pseudo-second-order kinetics model were closer to 1 and higher than that of pseudofirst-order kinetics model, indicating that complex physical-chemical adsorption played an important role in the process. It can be attributed to the fact indicating that the pseudo-first-order and pseudo-second-order kinetic models can better describe the adsorption behavior of toluene on OH-ZSM-5 and ZG-15%, respectively.
Figure 10 shows the intraparticle diffusion model of toluene adsorption on OH-ZSM-5 and ZG-15%. The toluene adsorption curves can be divided into three stages at different temperatures. Each stage of the fitting curve did not pass through the origin, and theR2was lower, indicating that the intraparticle diffusion was not the dominant role of adsorption, and there were other influencing factors. For OH-ZSM-5, the adsorption was mostly affected by the pore size and micro-mesoporous structure. However, for ZG-15%, the micro-mesoporous content, π-π bonds and H-bond structures provided more adsorption sites for toluene molecules. The third stage is the slow adsorption stage. With the decrease of adsorption sites, the adsorbent material slowly adsorbs toluene to saturation.
4.6 Adsorption mechanism
Figure 11 shows the schematic diagram of toluene adsorption mechanism on OH-ZSM-5 (a) and ZSM-5@GO (b) composite. Toluene is a small polar molecule.Therefore, the process of adsorption mainly occurs on the surface of adsorbent and micro-mesopores[38-39].In Figure 11(a), the specific surface area and micromesopore content of ZSM-5 was increased by NaOH etching treatment, and thereby more adsorption sites were formed to adsorb small toluene molecules. Therefore, the main adsorption mechanism of OH-ZSM-5 is pore-filled physical adsorption. However, in Figure 11(b), small toluene molecules were adsorbed in micro-mesoporous structures of ZSM-5@GO through pore filling adsorption.In addition, there were many structures similar to large π bond in GO of composite surface, which could form π-π interaction with π-π bond in benzene ring to adsorb toluene[40-41], and the surface functional group (such as-COOH) could form H-bond with the methyl functional group contained in toluene to enhance the toluene adsorption performance[42-43]. The composite had a higher adsorption capacity, indicating that low temperature could promote adsorption.
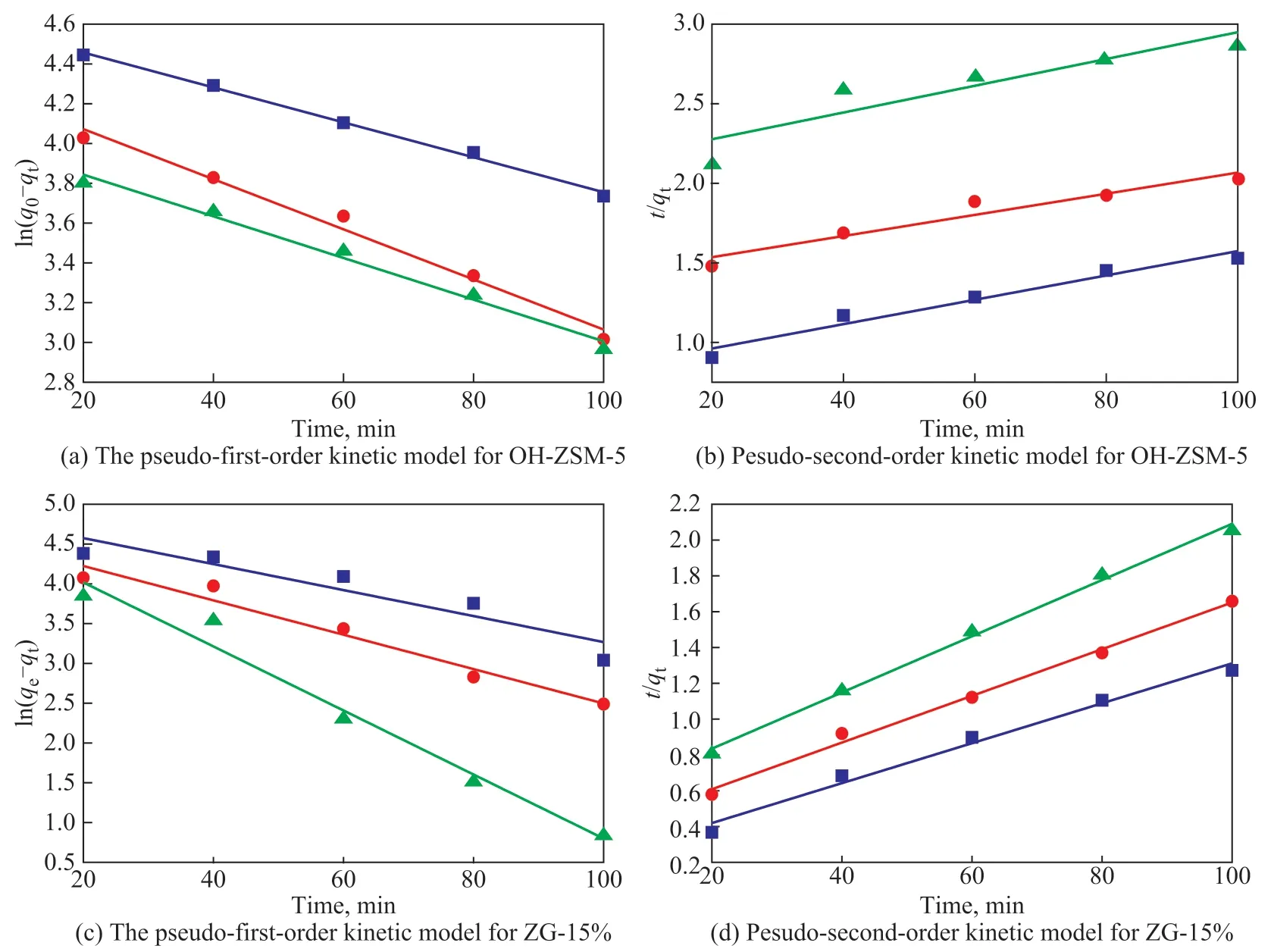
Figure 9 Kinetic model for toluene adsorption(The toluene initial concentration and adsorbent mass were set at 1 300 mg/m3 and 0.3 g and the temperature was set at 25 °C, 35 °C, 45 °C)
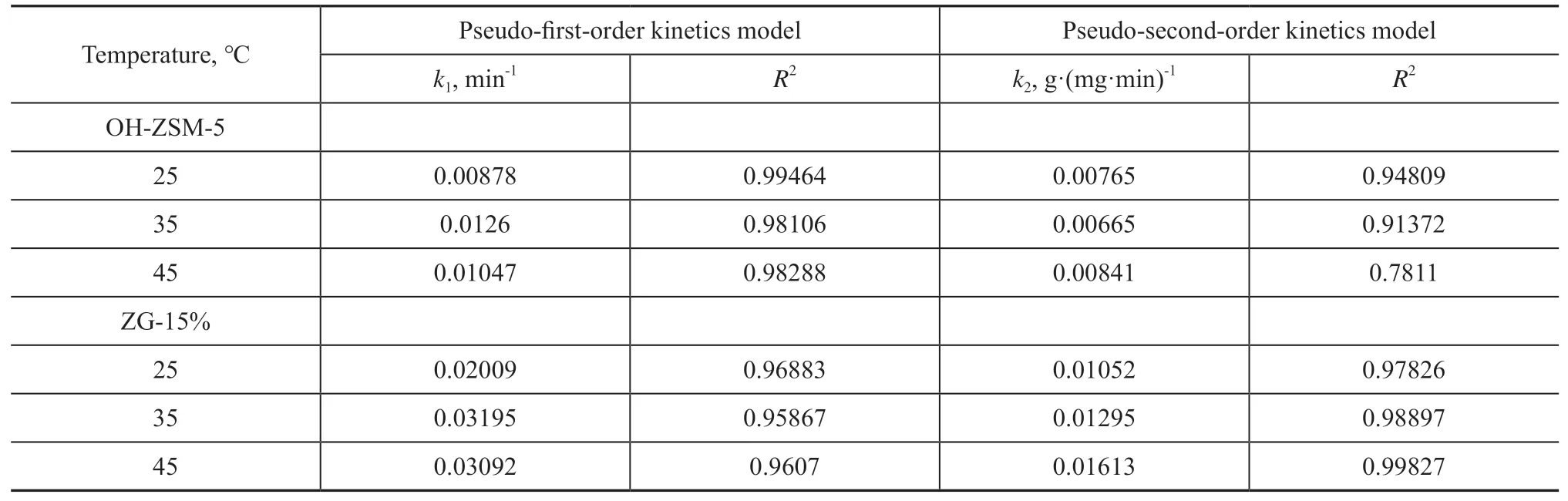
Table 3 Kinetic parameters of toluene adsorption at different temperatures
5 Conclusions
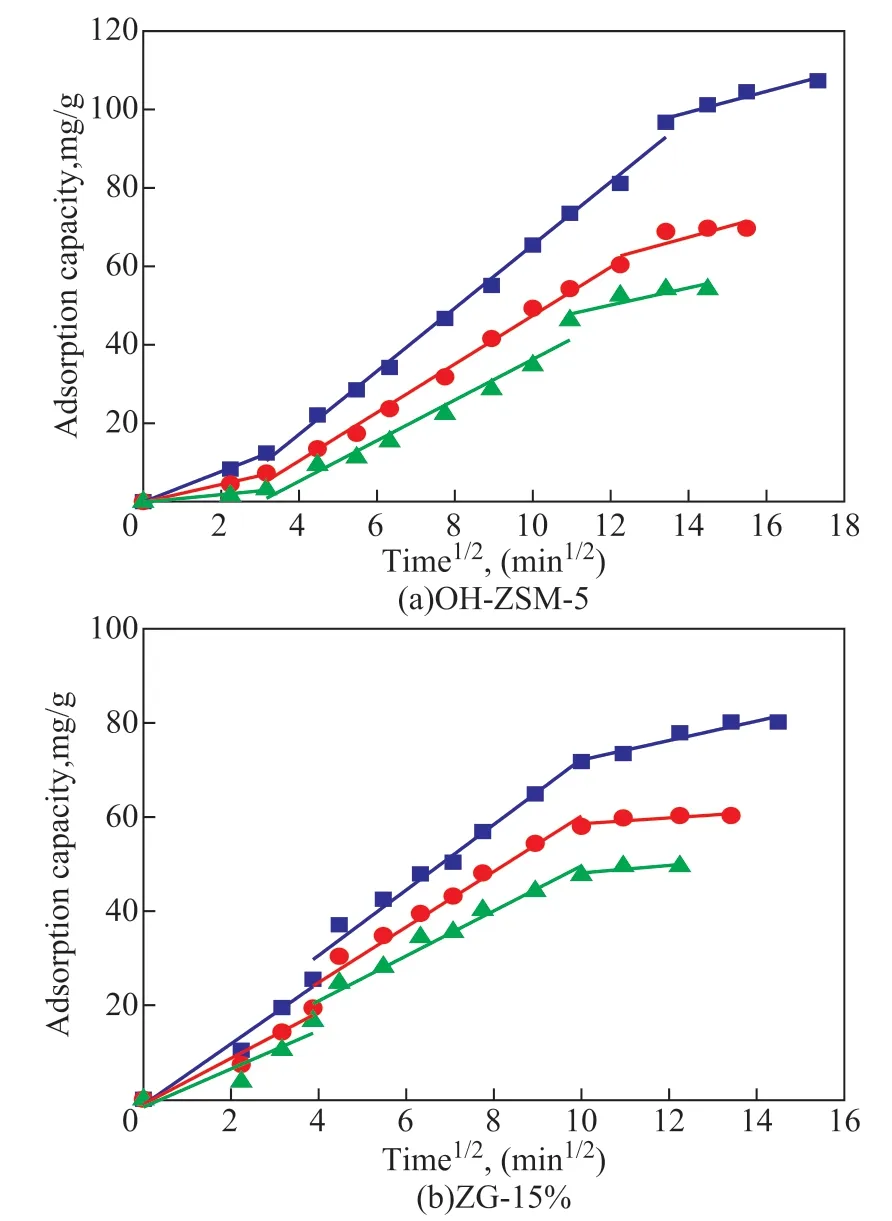
Figure 10 Intraparticle diffusion model of toluene(The toluene initial concentration and adsorbent mass were set at 1 300 mg/m3 and 0.3 g, and the temperature was set at 25 °C, 35 °C, 45 °C)
In this work, OH-ZSM-5 was prepared by NaOH solution immersion etching, which increased the specific surface area and micro-mesopore content of ZSM-5 zeolite to improve the adsorption performance. Moreover, GO was prepared by the improved Hummer’s method, and ZSM-5@GO was synthesized by the ultrasonic-assisted heating immersion method, the adsorption performance was improved by integrating the pore structure, specific surface area, large π-bonds, and the surface functional groups in GO. In the dynamic adsorption experiments,the toluene adsorption capacity of two adsorbtion materials were investigated under different conditions,which revealed that the toluene adsorption capacity was positively correlated with the initial toluene concentration and the adsorbent mass, but was negatively correlated with the temperature. The saturated adsorption capacity of OH-ZSM-5 (107.3 mg/g) was 1.34 times higher than that of ZG-15% (80.2 mg/g). The adsorption behavior of OHZSM-5 and ZSM-5@GO could be better described by the pseudo-first-order kinetic model and the pseudo-secondorder kinetic model, respectively.
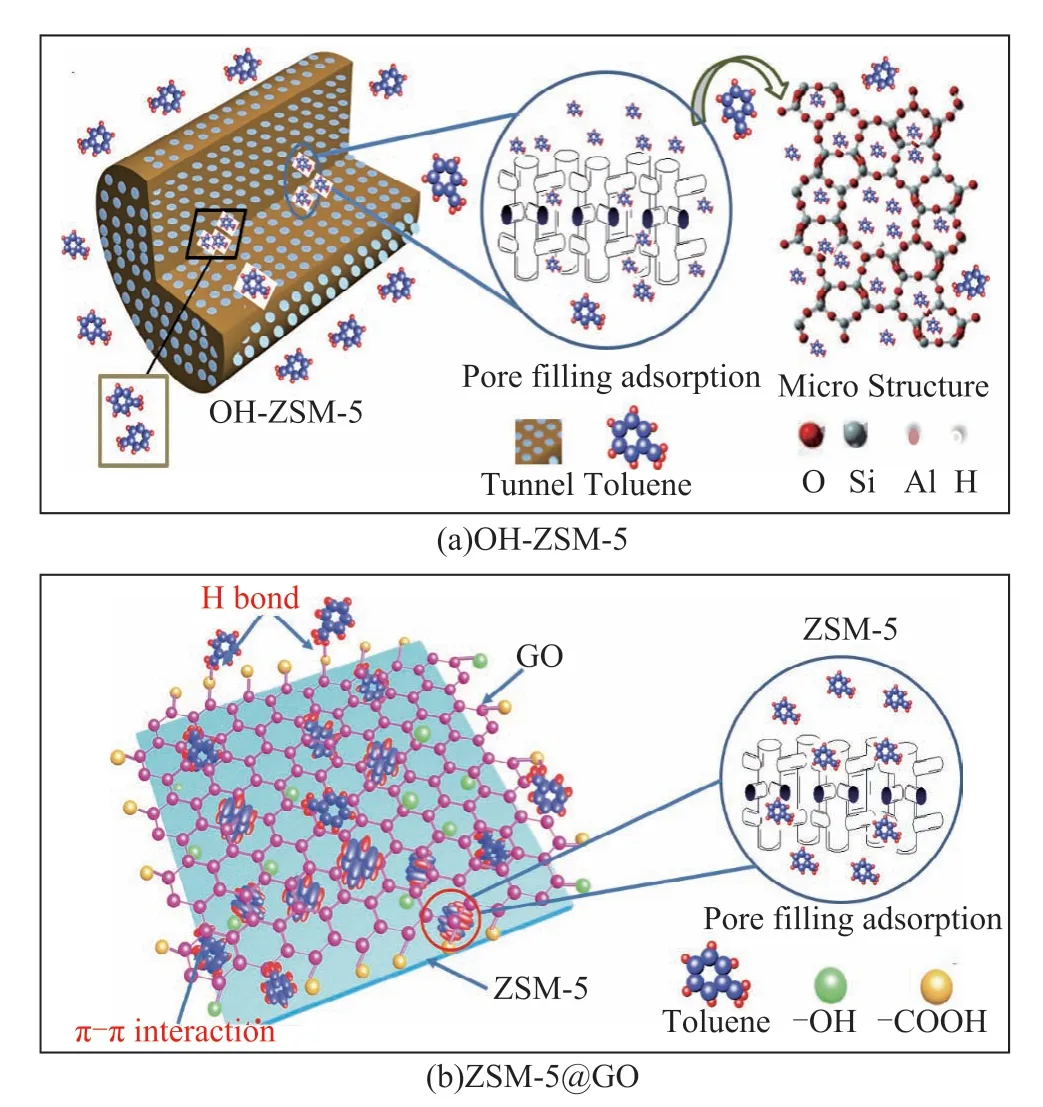
Figure 11 Schematic diagram of toluene adsorption mechanism

Table 4 Fitting parameters of intraparticle diffusion model
Acknowledgement:This research is financially supported by the Open Project Program of State Key Laboratory of Petroleum Pollution Control (No. PPCIP2017005).
- 中國(guó)煉油與石油化工的其它文章
- Preparation and Rheological Properties of Vacuum Lubricating Grease
- Effects of Different Plant Hormones for Microbial Degradation of PASHs and Diesel under Aerobic Conditions
- Nitrogen Removal Performance of Denitrifying Ammonium Oxidation System in Treating Sulfamethoxazole-laden Secondary Wastewater Effluent
- Removal of Basic Nitrogen Compounds from Diesel Fraction with NMP-0.5ZnCl2 Coordinated Ionic Liquid
- Flow Characteristics of Crude Oil with High Water Fraction during Non-heating Gathering and Transportation
- Effects of Operating Conditions on the Catalytic Performance of HZSM-5 Zeolites in n-Pentane Cracking

