Effects of Different Plant Hormones for Microbial Degradation of PASHs and Diesel under Aerobic Conditions
Shang Xiufang; Li Lin,2; Xiao Xinfeng; Xue Jianliang; Gao Yu; Gao Hongge; Hu Shugang
(1 College of Safety and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590;2 State Key Laboratory of Petroleum Pollution Control, China National Petroleum Corporation, Beijing 102206)
Abstract: The effects of plant hormones for biodegradation of polycyclic aromatic sulfur heterocycles (PASHs) and diesel fuel were studied. Indole butyric acid (IBA) and gibberellin were found to promote biodegradation of DBT and diesel,respectively. Concentrations of plant hormones, pH, temperature, soil moisture and substrate concentrations were optimized in microbial metabolic processes. Two main factors including temperature and IBA concentration were determined by factor analysis in DBT biodegradation. And soil moisture and diesel concentration were important factors in diesel biodegradation.Binding sites between cell surface and DBT or diesel components were performed by molecular operating environment(MOE). This study suggested that plant hormones could be applied to effectively remove pollutants in environment.
Key words: plant hormones; PASHs; diesel; bioremediation; oil-polluted soil
1 Introduction
In recent years, soil pollution has severely threatened security of the living environment[1]. With rapid development of oil industry[2-3], oil-contaminated soil has inevitably become a serious ecological and environmental problem. Polycyclic aromatic sulfur heterocvclics (PASHs), which have the sulfur atom in the heterocyclic ring, are more stable and biologically toxic than polycyclic aromatic hydrocarbons (PAHs) in crude oil[4]. Dibenzothiophene (DBT) and its derivatives are important components of PASHs with negative effects of carcinogenesis, teratogenesis and mutagenesis[5]. They are generally considered as the sulfur model compounds for biodesulfurization[6]. Removal of petroleum hydrocarbons and heterocyclic compounds from crude oil in soil is a difficult and long-term process. Microbial remediation is becoming a widely recognized technology featuring non-secondary pollution and fewer processing steps[7-8].Under the long-term stress of petroleum pollutants,microorganisms with degradation ability have been discovered, such asAeromonassp.,Bayerinckiasp.,Corynebacteriumsp.,Cyanobacteriasp.,Flavobacteriasp.,Micrococcussp.,Mycobacteriumsp.,Nocardiosp.,Pseudomonassp.,Rhodococcussp., etc.[9]
However, lower bioactivity, stability, and longer bioremediation period are key factors limiting further application of bioremediation. Liu[10]reported that diesel-degrading bacteria named as CC-CF3 and CCJG39 disappeared after 30 days and 90 days in situ bioremediation of contaminated soil, respectively.Rahman[11]also founded that oil biodegradation efficiency was only 20% after 20 days of treatment. Plant hormones are usually used in agricultural production for promoting plant growth and reducing the stress effects of heavy metals, such as Cd and Pb[12]. Indolebutyric acid (IBA)was reported that it was more effective in accelerating root growth compared with other auxins[13]. And an appropriate concentration of IBA was confirmed as an important factor for the processes of plants growth. Qu[14]showed that the CuAO zeta-derived reactive oxygen species was required for indole-3-butyric acid (IBA)-induced lateral root (LR) development. Gibberellins (GAs) are important endogenous phytohormones which can modulate many plant growth processes, including seed germination,flowering and forming germ[15]. Gibberellin promotes fungal entry and colonization during Paris-type arbuscular mycorrhizal symbiosis in eustoma grandiflorum[16].
Furthermore, plant hormones not only can promote growth and development of plants, but also can accelerate the cell division of microorganisms[17]. Therefore, there is a hypothesis that plant hormones can be applied in controlling the environmental pollutants, such as bioremediation of oil-contaminated soil. Finally,gibberellin and IBA were chosen as biological assistants for degrading bacteria to remove DBT and diesel.Furthermore, the environment factors, such as pH value,soil moisture content, concentration of substrates and temperature, were also investigated in our study. And key factors affecting the biodegradation of pollutants were determined by using SPSS software. In addition,molecular operating environment (MOE) was performed to determine the possible binding sites between DBT and diesel components with cell surface.
2 Materials and Methods
2.1 Culture medium and chemicals
Basal salts medium (BSM) was composed of 0.6 g/L of Na2HPO4, 0.2 g/L of KH2PO4, 4.0 g/L of NaNO3,0.01 g/L of CaCl2, 0.01 g/L of FeSO4, 0.3 g/L of MgSO4,and 0.5 g/L of yeast extract. Dibenzothiophene (with a purity of 98%) was purchased from Aladdin (Shanghai,China). Gibberellin (0.1 g) was dissolved in 100 mL of deionized water with 0.5 mL of ethyl alcohol to form a final gibberellin concentration of 1 g/L. Similarly, IBA(0.01 g) was dissolved in 100 mL of deionized water with 0.6 mL of ethyl alcohol to form a final IBA concentration of 0.1 g/L. All the other reagents were of analytical grade and commercially available.
2.2 Bacterial sources
ThePseudomonassp. LKY-5 which utilized dibenzothiophene as a sole carbon and energy source had been isolated from the oil-contaminated soils in our previous work[18]. Strain LKY-5 was cultivated for 16 hours in a shaking incubator at 30 °C under a oscillating rate of 160 r/min. An outstanding strain 1-3 was obtained and identified asPseudomonasgenus after two months of screening. The colonies of strain 1-3 are faint yellow and jagged. Cells are gram-negative, non-spore-forming and having motility. Gelatin liquefaction and catalase reactions are positive.Pseudomonassp. 1-3 was cultivated for 20 hours in a shaking incubator (with the bacteria density equating to 3.4×107CFU/mL).
2.3 The degradation of DBT by strain LKY-5 with different plant hormones
DBT was added into the basal salts medium with a final concentration of 120 mg/L. The inoculation amount ofPseudomonassp. LKY-5 was 10% (vol/vol). Gibberellin and IBA were added into BSM with the concentration in the range of 0.5―7 mg/L. All experiments were performed in a rotary shaker under a oscillating rate of 160 r/min with temperature of 30 °C for 7 days. Besides, gibberellin and IBA were added into the soils with the concentration ranging from 0.5 mg/kg to 7 mg/kg. The soil moisture was maintained at 15%. All the experiments were performed in a constant temperature incubator at 30 °C for 7 days. The analysis of DBT can been found in our previous work[18].
2.4 Degradation of diesel by strain 1-3 with different plant hormones
Different concentrations of gibberellin (0.02 — 2 mg/L)and IBA (0.5 — 5 mg/L) were placed in BSM for biodegradation process. Gibberellin (0.02 — 2 mg/kg)and IBA (0.5 — 5 mg/kg) were also added into soils which had been screened by a 2-mm sieve. The samples were extracted by petroleum ether (at 30 — 60 °C) in an ultrasonic cleaner for 10 min. The absorbance of supernatant was measured at 255 nm with an ultraviolet spectrophotometer.
2.5 Effects of pH, temperature, substrate concentration and moisture on biodegradation
The effects of pH value (6 — 8.5), soil moisture content(5% — 25%), diesel concentration [(10 — 1000) mg/30 g soil] and temperature (15 — 40 °C) on the degradation of diesel with added gibberellin coupled withPseudomonassp. 1-3 were investigated. Effects of DBT concentration(120 — 500 mg/kg), pH value (6 — 8.5), soil moisture content (5% — 25%), and temperature (15 — 40 °C)were also studied. The SPSS 18.0 software was used for selecting important indicators in biodegradation of diesel and DBT. The component matrix was established to extract principal factors. In order to obtain a comprehensive factor, the ratio of the contribution rates of two main factors was weighted and summarized, and the comprehensive factor was obtained as:
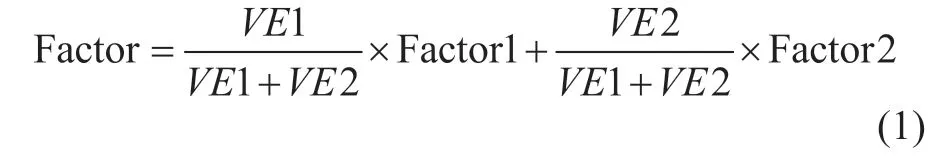
where VE1 and VE2 represent variance related with component 1 and component 2, respectively.
2.6 Molecular docking simulation
The molecular operating environment (MOE) is a comprehensive software system in pharmaceutical and life sciences. Rhamnolipids produced byPseudomonassp. LKY-5 were analyzed for enhancing bioavailability of pollutants in our previous work[19]. Then, MOE software was used to simulate the binding sites between rhamnolipid macromolecule with DBT and diesel includingn-hexadecane, 3,5-dimethylphenol, and cyclohexyl-2-propanone. The potential low-energy binding sites were found by the site finder module.Finally, internal interactions within these complexes were visualised by MOE ligand module.
3 Results and Discussion
3.1 Biodegradation of DBT by strain LKY-5 in the presence of gibberellin and IBA
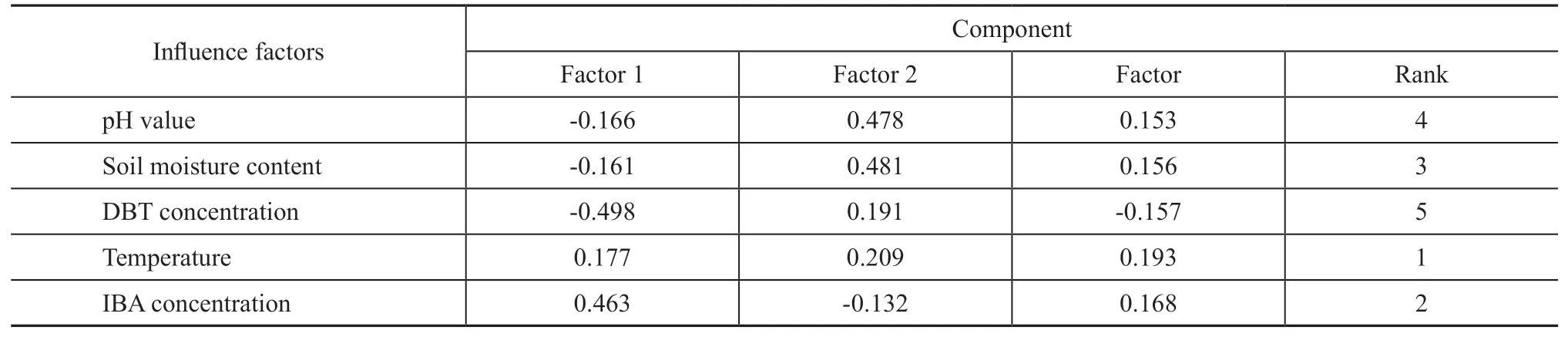
The effects of different concentrations of gibberellin and IBA on the degradation of DBT by strain LKY-5 were investigated. In Figure 1 (a), gibberellin and IBA promoted microbial metabolism in the aqueous environment. The degradation rate had reached 81%with addition of 2 mg/L of gibberellin. Similarly, the degradation of DBT was enhanced by IBA (2 — 7 mg/L).And the biodegradation was the highest (92%), when IBA concentration was 5 mg/L. In Figure 1 (b), DBT degradation was also significantly increased in the presence of gibberellin and IBA. However, biodegradation was not favorable in soils. The optimal concentration of IBA by strain LKY-5 was 7 mg/kg for DBT degradation.The degradation of DBT with addition of IBA was found to be efficient. Therefore, effects of pH value, moisture,substrate concentration, and temperature on DBT degradation were confirmed in soils. It can be seen from Figure 2 (a) that DBT was more effectively degraded in neutral or alkaline soils. The rate of DBT removal was the highest (69%) at a pH of 7.5 after 7 days. Figure 2 (b) shows that a soil moisture of 15% was suitable for biodegradation of DBT. When the soil moisture was 25%, the degradation of DBT was inhibited obviously. In this case the soil voids were filled with excess moisture, and oxygen was inadequate for microbial growth. Figure 2 (c and d) shows that the biodegradation gradually reduced with an increasing DBT concentration. The degradation of DBT was the highest(64%) at a DBT concentration of 120 mg/kg. And bioactivity of strain LKY-5 was the best at 30 °C.
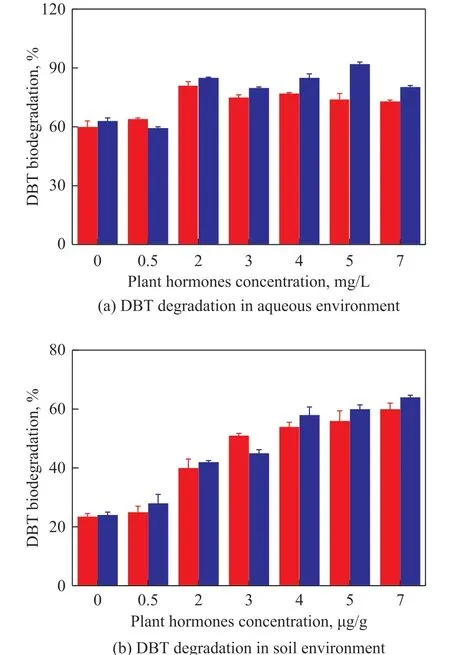
Figure 1 Effects of different concentrations of gibberellin and IBA on DBT degradation by Pseudomonas sp. LKY-5 in aqueous and soil environment
3.2 Analysis of factors influencing DBT degradation
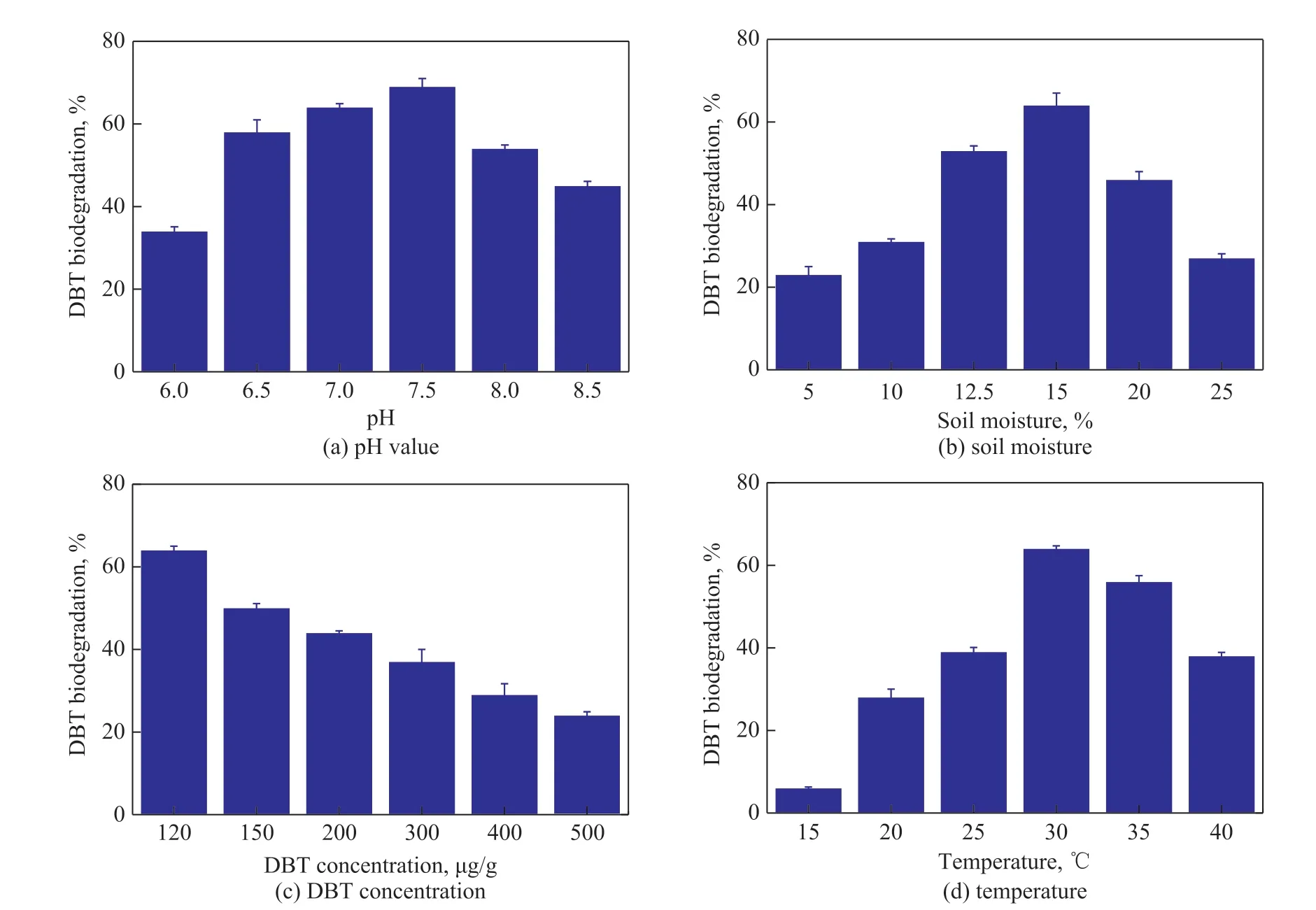
Figure 2 Effects of different environmental factors on DBT degradation by Pseudomonas sp. LKY-5 with addition of IBA to soils
Analysis of factors including pH, soil moisture, DBT concentration, temperature, and IBA concentration was conducted. The result of KMO test was 0.636 which was greater than the standard of 0.5. And the Barelett test showed that the significance probability was 0.01,denoting that there was little difference in the correlation matrix. Therefore, the factor-analysis method is feasible.Eigenvalue and total variance explained are indicated in Table 1 (a). The accumulative total variance explained was 96.521%. The two principal factors reserved 96.521%of original index variable. And their eigenvalues were 3.620 and 1.206, which were greater than the standard of 1. Therefore, it is appropriate to choose two principal factors. The variance explained for the first public factor was 48.696%, whereas the second public factor was 47.825%. In Table 1 (b), scores of Factor 1 and Factor 2 were obtained by SPSS. And a comprehensive factor was obtained from formula (1). Overall, the sequential scores of five factors were temperature, IBA concentration,soil moisture, pH value and DBT concentration. Hence,temperature and IBA concentration were considered as the main factors in DBT degradation.
3.3 Biodegradation of diesel by strain 1-3 in the presence of gibberellin and IBA
Figure 3 (a) shows that biodegradation of diesel was the highest (51%) with the addition of gibberellin (0.05 mg/L). High concentration of gibberellin (>1 mg/L) was confirmed to be capable of inhibiting the degradation of diesel. Aihebaiers[13]has also suggested that high plant hormone concentrations may interfere metabolism of microbial protein and nucleic acid. The effect of IBA was not obvious as compared with gibberellin (Figure 3 (b)). The highest degradation rate was only 26% at an IBA dosage of 1 mg/L. In soils, the diesel removal rate was 68% at a gibberellin concentration of 0.05 μg/g(Figure 3 (c)). Similarly, the degradation of diesel gradually increased from 18% to 37% with an increasing IBA concentration (from 0 μg/g to 2 μg/g) as shown in Figure 3 (d). It is interesting that the degradation of diesel in soils is generally higher than that in aqueous environment, although the same levels of gibberellin or IBA were added. Furthermore, the degradation of diesel by gibberellin was more effective than IBA. Wang[20]reported that gibberellin was applied as the soil drench for increasing indigenous microbial cells. Moreover,gibberellin and IBA were also reported as capable of promoting root growth and cell division[21-22]. The above discovery is beneficial to decontaminating the petroleum polluted soils.
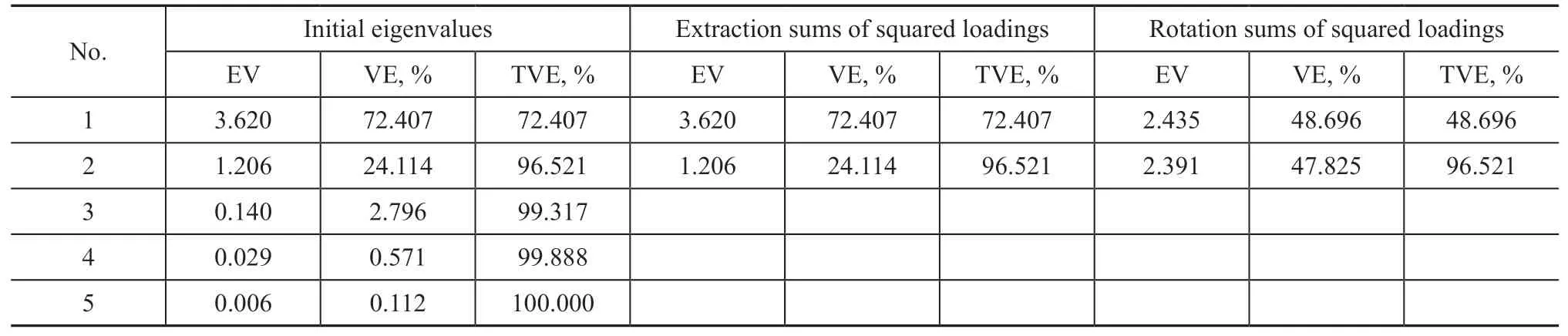
Table 1 Factor analysis in DBT degradation(a) Total variance explained
(b) Scores and ranking
In Figure 4 (a), biodegradation of diesel gradually increased with an increase of pH value from 6 to 7.5. The degradation of diesel was the highest (68%) at a pH value of 7.5. Figure 4 (b) shows that biodegradation of diesel was the highest (75%) at a soil moisture of 12.5%. The biodegradation was then highest after adding 100 mg of diesel into 30 g of soils (Figure 4 (c)). The microbial activity was inhibited with higher concentration of pollutants. In Figure 4 (d), the temperature of 30 °C was suitable for biological metabolism of diesel. The bioactivity of this strain was irreversibly damaged at higher temperature.
3.4 Analysis of factors influencing diesel degradation
The procedures of factor analysis in diesel degradation were the same as DBT degradation. The result of KMO test was 0.567. The Barelett test showed that the significance probability was 0.006 (<0.05). In Table 2 (a),the accumulative total variance explained was 95.579%.And their eigenvalues were 3.618 and 1.161, respectively.The variance explained of the first public factor was 55.068%, whereas the second public factor was 40.511%.Table 2 (b) shows that the sequential scores of five factors were soil moisture, diesel concentration, temperature,pH value, and gibberellin concentration. Therefore, soil moisture and diesel concentration were considered as the main factors in diesel degradation study.
3.5 Molecular docking simulation between DBT or diesel components and cell surface
Docking studies were carried out on DBT and diesel components (n-hexadecane, 3,5-dimethylphenol and cyclohexyl-2-propanone) via molecular operating environment. Figure 5 (A, B) and (a, b) shows the docking structures and composite conformations on DBT andn-hexadecane, respectively. Hydrophobic and electrostatic interactions[23]were formed between DBT and key residues such as Ser A96, His A202, Met A199,Tyr A162, Thr A200, Arg A194, Glu A157, and Gln A158.Hydrophobic and electrostatic interactions were formed betweenn-hexadecane and the key residues such as Ser A148, Gly A193, Gly A147, Pro A192, Val A149, Arg A194, Tyr A162, Thr A200, Met A199, Ser A197, Arg A19, Gly A20, lle A21, Asn A92, and Lys A166. However,weak bonds were formed between rhamnolipid and DBT orn-hexadecane. The production of weak bonds might be due to the principle of similar compatibility[24]. In Figure 5 (C and D), binding forces of rhamnolipid and 3,5-dimethylphenol or cyclohexyl-2-propanone were hydrogen bonds. The combination model with a lowest binding energy was selected from the various possiblestructures generated. The probable linked conformations are shown in Figure 5 (c and d), respectively. Hydrogen bonds with a length of 0.282 nm were formed between 3,5-dimethylphenol and the residue Thr A200. Hydrogen bonds with a length of 0.274 nm were formed between cyclohexyl-2-propanone and the residue Thr A162.
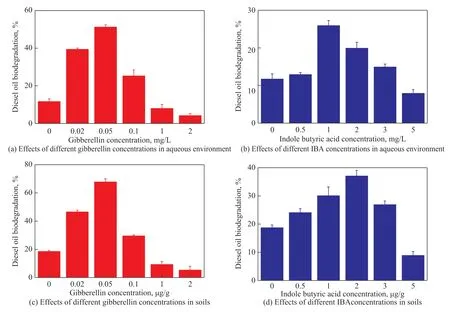
Figure 3 Effects of different concentrations of gibberellin and IBA for diesel degradation by Pseudomonas sp.1-3 in aqueous and soil environment
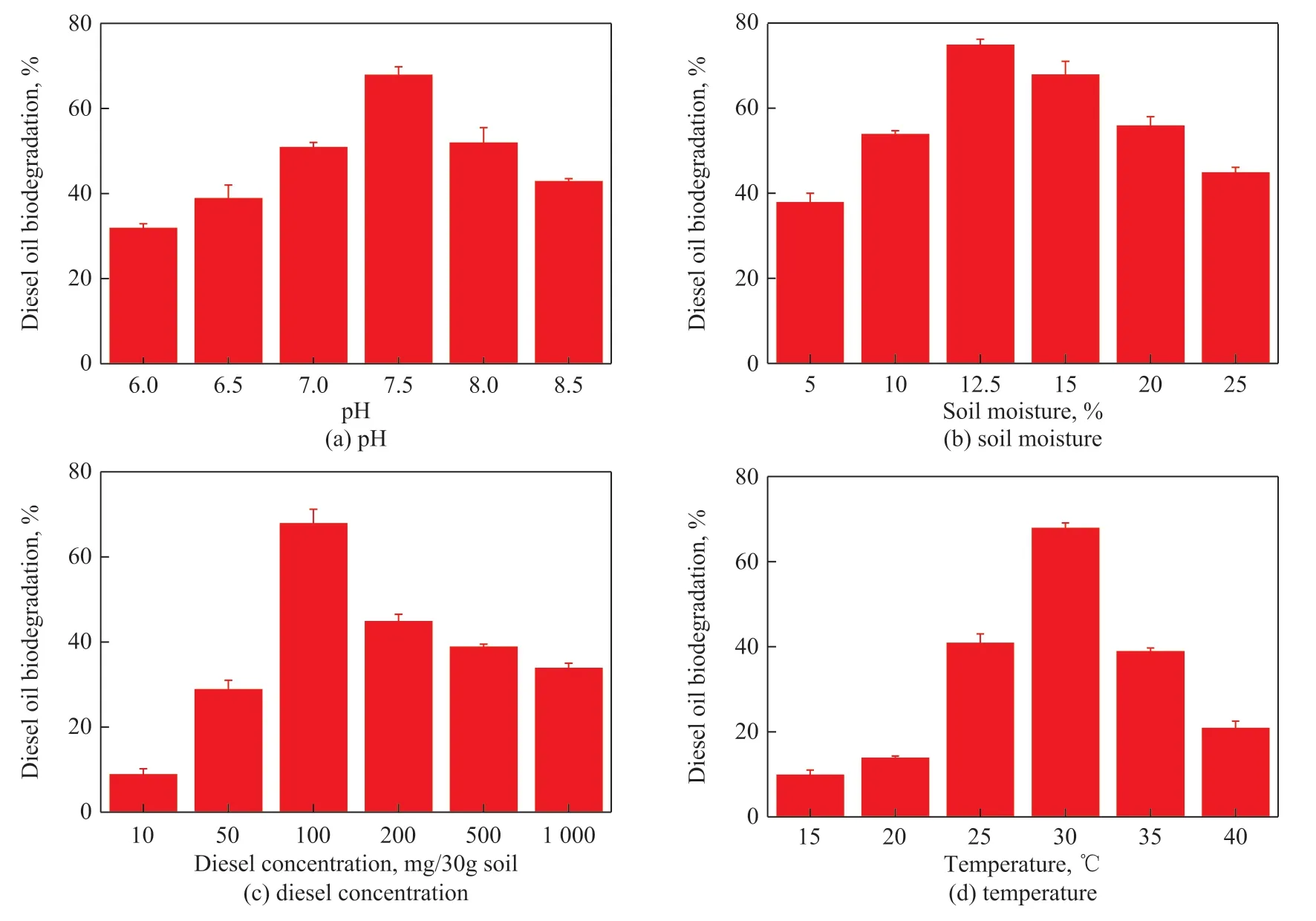
Figure 4 Effects of different environmental factors for diesel degradation by Pseudomonas sp.1-3 with addition of gibberellin in soils

(b) Scores and ranking
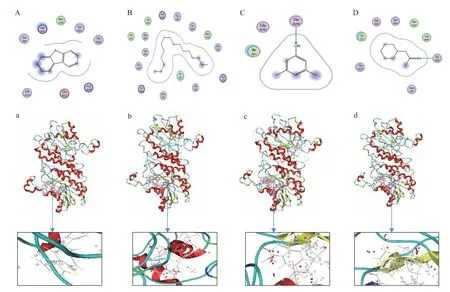
Figure 5 Molecular docking results of planar graph (A~D) and space diagram (a~d).(A: DBT; B: n-Hexadecane; C: 3,5-Dimethylphenol; D: Cyclohexyl-2-propanone)
4 Conclusions
IBA was more suitable for promoting the bioactivity ofPseudomonassp. LKY-5 as compared with gibberellin in DBT degradation. Biodegradation of DBT could be enhanced to 92% with an IBA concentration of 5 mg/L in aqueous environment. In soils, biodegradation of DBT was 64% with a IBA concentration of 7 mg/kg. In addition,a temperature of 30 °C, a soil moisture of 15%, a pH value of 7.5, and a DBT concentration of 120 mg/kg were favorable to biodegradation. And main factors including temperature and IBA concentration were determined by factor analysis. Differently, gibberellin was more suitable for promoting bioactivity of strain1-3 as compared with IBA in diesel degradation. Diesel biodegradation was 68% with a gibberellin concentration of 0.05 mg/kg. And a pH value of 7.5, a temperature of 30 °C, a soil moisture of 12.5%, and a diesel concentration of 0.33%(0.1 g/30 g) were favorable to the biodegradation of diesel.Soil moisture and diesel concentration were important factors for diesel biodegradation by factor analysis. In addition, molecular docking studies between DBT or diesel components and rhamnolipid were performed by MOE.It was found that main interactions between rhamnolipid and 3,5-dimethylphenol or cyclohexyl-2-propanone were hydrogen bonds. Hydrophobic and electrostatic interactions were found between rhamnolipid and DBT orn-hexadecane.
Acknowledgements:The authors gratefully acknowledge the financial support provided by the Natural Science Foundation of Shandong Province (Grant No. ZR2019BD035), the Open Project Program of State Key Laboratory of Petroleum Pollution Control (Grant No. PPC2017020), the CNPC Research Institute of Safety and Environmental Technology, and the Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (Grant No. 2016RCJJ016).
- 中國(guó)煉油與石油化工的其它文章
- Preparation and Rheological Properties of Vacuum Lubricating Grease
- Nitrogen Removal Performance of Denitrifying Ammonium Oxidation System in Treating Sulfamethoxazole-laden Secondary Wastewater Effluent
- Removal of Basic Nitrogen Compounds from Diesel Fraction with NMP-0.5ZnCl2 Coordinated Ionic Liquid
- Flow Characteristics of Crude Oil with High Water Fraction during Non-heating Gathering and Transportation
- Comparison and Analysis of Toluene Adsorption Properties of ZSM-5 Molecular Sieve Treated by Different Modification Methods: Adsorption Kinetic and Mechanism Studies
- Effects of Operating Conditions on the Catalytic Performance of HZSM-5 Zeolites in n-Pentane Cracking

