The role of peptidase neurolysin in neuroprotection and neural repair after stroke
Vardan T. Karamyan
Abstract Current experimental stroke research has evolved to focus on detailed understanding of the brain’s self-protective and restorative mechanisms, and harness this knowledge for development of new therapies. In this context, the role of peptidases and neuropeptides is of growing interest. In this focused review, peptidase neurolysin (Nln) and its extracellular peptide substrates are briefly discussed in relation to pathophysiology of ischemic stroke.Upregulation of Nln following stroke is viewed as a compensatory cerebroprotective mechanism in the acute phase of stroke, because the main neuropeptides inactivated by Nln are neuro/cerebrotoxic (bradykinin, substance P, neurotensin, angiotensin II,hemopressin), whereas the peptides generated by Nln are neuro/cerebroprotective(angiotensin-(1-7), Leu-/Met-enkephalins). This notion is confirmed by experimental studies documenting aggravation of stroke outcomes in mice after inhibition of Nln following stroke, and dramatic improvement of stroke outcomes in mice overexpressing Nln in the brain. The role of Nln in the (sub)chronic phase of stroke is less clear and it is likely, that this peptidase does not have a major role in neural repair mechanisms.This is because, the substrates of Nln are less uniform in modulating neurorestorative mechanisms in one direction, some appearing to have neural repair enhancing/stimulating potential, whereas others doing the opposite. Future studies focusing on the role of Nln in pathophysiology of stroke should determine its potential as a cerebroprotective target for stroke therapy, because its unique ability to modulate multiple neuropeptide systems critically involved in brain injury mechanisms is likely advantageous over modulation of one pathogenic pathway for stroke pharmacotherapy.
Key Words: compensatory cerebroprotection; drug target; endogenous neuroprotective mechanism; neuropeptide; neurorestoration; peptidase
Introduction
Based on current estimates, stroke is the leading and growing cause of long-term disability and the second cause of death worldwide. Despite important advances in stroke awareness and prevention, the use of new stroke guidelines and improved acute care in hospitals in the last decade, there is a substantial unmet need for development of new stroke treatments. As a consequence, traditional drug development approaches for stroke therapy are being reevaluated and the current translational stroke research focuses more on understanding the brain’s self-protective and repair mechanisms (Lo, 2008; Iadecola and Anrather,2011; Carmichael, 2016). The fundamental premise of this approach is that the brain has multifaceted central and peripheral protective mechanisms directed at maintaining tissue homeostasis following adverse conditions and injury.Therefore, it is important to elucidate and better understand the triggers and effectors of endogenous neuroprotection and neurorestoration, because such information could lead to development of therapeutic interventions which mimic or engage the self-protective and repair mechanisms of the brain for successful stroke therapy.
In this context, the role of neuropeptides in brain’s selfpreservation and restoration after stroke is of great interest(Nistor et al., 2018; Mousa and Ayoub, 2019). This is because neuropeptides are one of the most heterogeneous signaling molecules with neuromodulatory, neurotransmitter, and/or hormonal roles, which primarily function during adaptation/response of the nervous system to various challenges, and represent the so-called “l(fā)anguage of the stressed nervous system” (Hokfelt et al., 2003). Availability and (patho)physiological function of neuropeptides are directly controlled by peptidases, which generate and/or degrade bioactive peptides by catalytic hydrolysis of peptide bonds (Shrimpton et al., 2002; Karamyan and Speth, 2007; Speth and Karamyan,2008; Al-Badri et al., 2018). Notably, a number of experimental stroke studies have reported altered activity and/or expression of brain peptidases and related neuropeptide systems, and linked their function to pathophysiology of stroke (Rashid et al., 2014). In this brief review, a succinct summary of recent experimental studies originating from our laboratory and focusing on peptidase neurolysin (Nln) is provided to make a case that Nln is an endogenous compensatory and cerebroprotective mechanism in the acute phase of stroke.In addition, based on unpublished observations from our laboratory, and published studies from other research groups, the potential function of Nln in neurorestoration and functional recovery during the chronic phase of stroke is discussed.
Search Strategy and Selection Criteria
PubMed search was used with default settings and no restrictions. Each peptide substrate of neurolysin was searched in combination with neuroprotection, ischemia/stroke, neuroinflammation, vascular permeability, brain edema, cell death, angiogenesis, neurogenesis, neural plasticity, long-term potentiation, neural repair, glutamatergic excitatory neurotransmission, cognition and memory.
Peptidase Neurolysin and Its Extracellular Substrates
Nln (EC 3.4.24.16) is a zinc endopeptidase containing a His-Glu-X-X-His domain, that belongs to the most studied family of peptidases (M3 family) involved in processing of extracellular bioactive peptides expressed by neurons and glial cells(Shrimpton et al., 2002; Wangler et al., 2012, 2016). Nln is widely distributed in the central nervous system (Karamyan and Speth, 2008), and is predominantly found in a soluble form in the cytoplasmic domain. However, depending on the cell type, Nln can also be secreted, bind to the plasma membrane, or localize to mitochondria. Several studies have shown that Nln faces the outer side of the plasma membrane in neurons and is accessible to ligands outside of the cell(Vincent et al., 1996; Rashid et al., 2010), making extracellular substrates of Nln the primary focus of biochemical and pharmacological studies associated with this peptidase. On the contrary, the role of Nln in intracellular/mitochondrial processing of peptides is less explored but is likely very important for pathogenesis of certain neurological disorders(Rashid et al., 2014; Teixeira et al., 2018).
Neurotensin, bradykinin, substance P, angiotensin I, dynorphin A(1-8), metorphamide and hemopressin are the most characterized extracellular substrates of Nln (Shrimpton et al.,2002; Wangler et al., 2016). These peptides are hydrolyzed and inactivated by Nln, with the exception of dynorphin A(1-8), metorphamide and angiotensin I, which are converted into biologically active peptides Leu- and Met-enkephalins and angiotensin-(1-7), respectively. In addition, there is some evidence that Nln can also hydrolyze angiotensin II and somatostatin into inactive fragments (Rashid et al., 2014).Notably, all Nln substrates and generated active peptides are critically involved in pathogenesis of stroke (Rashid and Karamyan, 2018; Jayaraman et al., 2019; Karamyan, 2019),however their function during acute and chronic phases of stroke are likely different.
Neurolysin and Its Substrates during the Acute Phase of Stroke
A recent study from our laboratory conducted in the middle cerebral artery occlusion model of stroke (MCAO,1 hour of occlusion) revealed about two fold upregulation of membrane-bound Nln in ischemia-affected parts of the mouse brain 24 hours after reperfusion (Rashid et al.,2014). Notably, this upregulation was sustained in ischemiaaffected cerebral cortical areas for at least one week following stroke. Our observations indicated lack of transcriptional or translational regulation of Nln upregulation, and pointed out to dependence of Nln translocation from cytosol to the membranes and mitochondria in this process (Rashid et al.,2014). To understand the function of Nln in the post-stroke brain and its potential role in the brain’s response to stroke,in a subsequent study a two-pronged approach, inhibition and over-expression of Nln, was used and stroke outcomes were evaluated in the same mouse MCAO model of stroke(Jayaraman et al., 2019). Treatment of mice with a small molecule, specific inhibitor of Nln (Agaricoglyceride A) 1 hour after stroke, resulted in dose-dependent aggravation of injury measured by increased cerebral infarction and edema formation, blood-brain barrier dysfunction, elevation of certain cytokines, and motor and neurological deficit 24 hours after stroke. In this setting, administration of Agaricoglyceride A was accompanied with inhibition of Nln in the infarcted hemisphere and elevated levels of Nln substrate peptides neurotensin, substance P and bradykinin. In a reverse complementary approach, an adeno-associated viral construct for full-length Nln (AAV2/5-CAG-Nln) was used to overexpress the peptidase in the mouse brain. Decreased levels of neurotensin, substance P and bradykinin were documented in these conditions. Fourteen days afterin vivotransduction of Nln using AAV2/5-CAG-Nln, mice were subjected to stroke and the same outcome measures, used for the Agaricoglyceride A experiments, were evaluated 72 hours after stroke. Our observations revealed that abundance of Nln in the brain afforded profound cerebroprotection after stroke. Based on these two studies, it was concluded that upregulation of Nln during the acute phase of stroke is one of the brain’s compensatory and self-protective mechanisms directed towards inhibition of injury and restoration of brain functions after ischemia (Karamyan, 2019).
To better understand the (patho)physiological importance of Nln upregulation in the acute post-stroke brain it is important to recognize the function of Nln substrates soon after ischemic injury. Numerousin vivostudies have demonstrated involvement of bradykinin in stroke injury revealing the role of bradykinin receptors, both B1 and B2, in development of stroke-induced cell death, cerebrovascular permeability, cerebral edema and neurogenic inflammation(Albert-Weissenberger et al., 2013; Dobrivojevic et al.,2015). Pathological role of substance P in post-stroke neuroinflammation, oxidative stress, cerebrovascular permeability and edema formation has been demonstrated by different research groups in experimental studies using small molecule NK-1 receptor antagonists in rodents (Sorby-Adams et al., 2017; Richter et al., 2018). In addition, NK-1 receptorindependent neuroinflammatory and neurodegenerative effects of substance P have been documented recently(Wang et al., 2014; Green et al., 2019). Deleterious function of neurotensin in the setting of ischemia is supported by experimental studies documenting decreased survival of primary neurons after OGD/re-oxygenation (60 minutes of oxygen and glucose deprivation followed by 24 hours of reoxygenation) in the presence of neurotensin, and blockade of this effect by neurotensin receptor 1 antagonist SR48692(Antonelli et al., 2008; Ferraro et al., 2009). Notably, these actions of neurotensin likely involve enhanced N-methyl-D-aspartate receptor-mediated glutamate signaling in neurons (Antonelli et al., 2004; Kempadoo et al., 2013),which could exacerbate excitotoxicity following ischemia. In addition, there is experimental evidence that neurotensin,as a proinflammatory cytokine, enhances cerebrovascular permeability and neuroinflammation (St-Gelais et al., 2006),which in part, is mediated through degranulation of mast cells and release of inflammatory mediators (Theoharides,2017). It is important to note that hypothermia-mediated neuroprotective potential of neurotensin receptor agonists has been documented in severalin vivostudies (Choi et al.,2012; Lee et al., 2016). The latter indicates that stimulation of neurotensin receptors in the hypothalamic thermoregulatory center and subsequent reduction of core body temperature counteract pathological effects of NT1 receptor stimulation in brain regions directly affected by ischemia.
Among peptides generated by Nln, angiotensin-(1-7), formed from inactive precursor angiotensin I, is well-known for its neuroprotective and anti-inflammatory effects, which were also demonstrated in the setting of acute stroke in experimentalin vivostudies (Jiang et al., 2013; Bennion et al., 2015). Complementary to this, Nln converts endogenous opioids metorphamide and dynorphin A(1-8) into Metand Leu-enkephalins with potent delta-opioid receptor agonistic activity. Stimulation of delta-opioid receptors leads to enhanced ischemic tolerance and neuroprotection after stroke as documented by us and other research groups (Yang et al., 2015; Subedi and Wang, 2020). The last Nln substrate with relevance to stroke is hemopressin, which is a potent cannabinoid CB1 receptor inverse agonist, i.e. leads to blockade of CB1 receptor and inhibits its constitutive activity.Inactivation of hemopressin by Nln enables stimulation of the CB1 receptor, which appears to afford neuroprotection through inhibition of excitotoxicity and oxidative stress, and induction of hypothermia and long-term depression (van der Stelt and Di Marzo, 2005; Lara-Celador et al., 2013).
It is noteworthy that a small number of experimental studies contradict the described cerebrotoxic effects of some Nln substrates in the acute stroke setting (Amadoro et al., 2007;Albert-Weissenberger et al., 2013). However, several clinical studies support the evidence of cerebrotoxicity and strongly correlate severity and mortality of stroke and related acute neurodegenerative disorders to the elevated levels of bradykinin, neurotensin and substance P (Zacest et al., 2010;Kunz et al., 2013; Januzzi et al., 2016; Lorente et al., 2016).
Neurolysin and Its Substrates during the Chronic Phase of Stroke
Currently, limited experimental data is available about the state and function of Nln in the chronic phase of stroke.Our earlier study in the mouse transient MCAO model documented upregulated levels of Nln on day 7 after stroke(Rashid et al., 2014), which is considered the beginning of the chronic phase in mice (Syeara et al., 2020). This observation was also confirmed in mouse primary cortical neurons subjected to oxygen-glucose deprivation and re-oxygenation for 6 days (Rashid and Karamyan, unpublished observations).In addition, we have some preliminary observations in the mouse photothrombotic model of stroke (Alamri et al.,2018), indicating that upregulation of Nln may sustain after 7 days post-stroke through the chronic phase (Jayaraman and Karamyan, unpublished observations). This question is currently being investigated in our laboratory, and detailed information about the state of Nln and its main peptide substrates in the chronic phase of stroke should emerge in the future.
Unfortunately, not much is known about brain levels of Nln peptide substrates and their specific receptors in the chronic phase of stroke. However, a number of experimental studies provide information about specific function(s) of Nln substrates in the brain, which may potentially link them to the central mechanisms of neurorestoration and functional recovery in the setting of chronic stroke. For example, proangiogenic effects of neurotensin have been documented in a number of experimental studies (Bakirtzi et al., 2016; Mouritzen et al.,2018) suggesting its potential in neurorestorative mechanisms in the post-stroke brain. In addition, it is recognized that neurotensin augments glutamate release and transmission in certain brain areas (Antonelli et al., 2004; Zhang et al., 2015),which could also facilitate neural repair during recovery phase of stroke (Lo, 2008; Carmichael, 2016). Recentin vivostudies documented that activation of neurotensin NT1 receptor facilitates neuronal excitability and spatial learning and memory in rodents (Keiser et al., 2014; Xiao et al., 2014). This mechanism also, if activated in the setting of chronic stroke,could potentially enhance neural repair and restoration(Carmichael, 2016). Interestingly, another substrate of Nln,substance P also appears to be involved in glutamate release and mechanisms governing memory and learning (Yu et al., 2014b; Hertler et al., 2017). In addition, several studies have documented enhanced neurogenesis (Park et al.,2007; Yang et al., 2017), angiogenesis (Um et al., 2017; Lee et al., 2018; Mouritzen et al., 2018) and M2 polarization of macrophages (Leal et al., 2015; Lim et al., 2017) in response to substance P, pointing out to additional mechanisms through which this neuropeptide could potentially facilitate neural repair and functional recovery in the setting of chronic stroke. For bradykinin, the experimental evidence is somewhat contradictory. From one hand, a number of studies documented enhanced neurogenesis (Trujillo et al., 2012; Pillat et al., 2016), angiogenesis (Liesmaa et al.,2009; Yu et al., 2014a) and glutamatergic signaling (Kohno et al., 2008; Liu et al., 2009) induced by bradykinin, however others reported improvement of memory and learning upon blockade or deletion of bradykinin B1 receptor (Lemos et al.,2010; Bitencourt et al., 2017). Among angiotensin peptides,experimental studies indicate that angiotensin-(1-7),generated from angiotensin I by Nln, and its Mas receptor are involved in processes modulating neurogenesis (Freund et al., 2014; Garcia-Garrote et al., 2019), angiogenesis (Jiang et al., 2014; Zhao et al., 2015) and cognition/memory (Ho and Nation, 2018; Hay et al., 2019). For angiotensin II, which could accumulate in conditions of low Nln activity, there appear to be conditions in which it may impair processes related to memory and learning (Kim et al., 2017; Trofimiuk et al.,2018) and be potentially deleterious for neural repair. On the contrary, angiotensin II may potentiate excitatory synaptic transmission (Barnes et al., 2003; Stern et al., 2016) and/or neurogenesis, angiogenesis and axonal plasticity (Munk et al., 2007; Li et al., 2008; Umschweif et al., 2014), and hence potentially facilitate neural repair. There is more supportive experimental evidence for the role of delta opioid receptors,which are stimulated by Leu- and Met-enkephalins (generated from dynorphin A(1-8) and metorphamide by Nln), in processes related to neural repair. For example, several studies documented that stimulation of the delta opioid receptor facilitates neurogenesis, neurite outgrowth (Narita et al., 2006; Georganta et al., 2013; Wang et al., 2016) and likely angiogenesis (Dai et al., 2010), whereas others observed inhibition of γ-aminobutyric acid transmission and induction of long-term potentiation through stimulation of the same receptor (Bramham and Sarvey, 1996; Zhang and Pan, 2012).Lastly, there is no direct evidence for the role of hemopressin,which blocks CB1 cannabinoid receptor and is inactivated by Nln, in mechanisms associated with neural repair. However,numerous studies documented that stimulation of CB1 receptors results in enhanced neurogenesis and angiogenesis(Pisanti et al., 2011; Xapelli et al., 2013) but also inhibits glutamatergic excitatory neurotransmission (Domenici et al.,2006; Haj-Dahmane and Shen, 2009).
To summarize, it is important to reiterate that many of the studies discussed in this section do not provide direct experimental evidence on the role of Nln substrates in neural repair and functional recovery after stroke. However, the noted studies offer mechanistic clues that these peptidergic systems may play a role in processes involved in post-stroke recovery and therefore could be modulated by Nln.
Concluding Remarks, Neurolysin and Transitioning from Injury into Repair after Stroke
It is well-documented that many pathways are triggered after stroke, with deleterious mechanisms outweighing neuroprotective ones in the beginning, and neural repair mechanisms prevailing during the early to mid-phases of chronic stroke (Carmichael, 2016; Hatakeyama et al., 2020).Unfortunately, most often the latter mechanisms are not sufficient for full recovery after stroke. In most cases, responses identified as deleterious during the acute phase transition into beneficial mechanisms for neuronal and vascular recovery(e.g., N-methyl-D-aspartate receptor-mediated glutamate signaling, matrix metalloproteinase-mediated proteolysis).
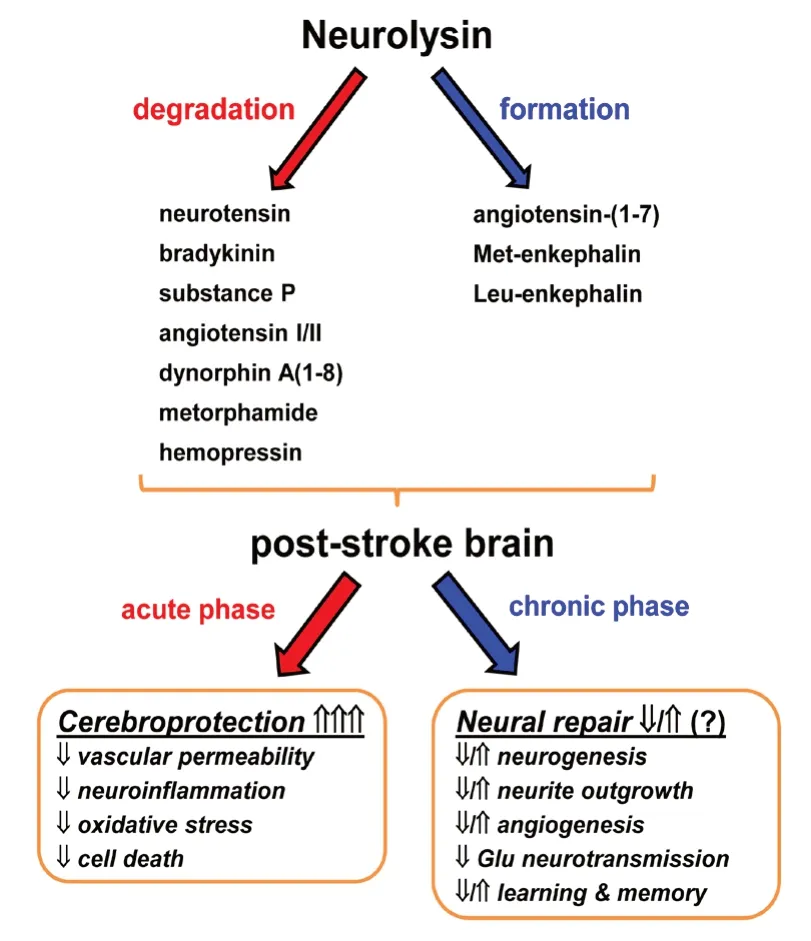
Figure 1 |Peptidase neurolysin (Nln) in the post-stroke brain.Nln, through its extracellular peptide substrates, appears to have more defined role in the acute phase of stroke and functions to protect the brain from ischemic injury. On the contrary, the role of Nln in the chronic phase of stroke is less understood and it is likely that this peptidase does not have a major function in neural repair mechanisms after stroke.
On the contrary, cellular processes which initially protect the brain from expansion of injury transition into harmful pathways during the period of recovery (e.g., γ-aminobutyric acid inhibitory signaling). Based on this biphasic nature of protective and deleterious mechanisms, it is possible that compensatory neuroprotective function of Nln during the acute and sub-acute phases of stroke, could transition into activity that hinders neural repair processes during later phases of stroke. From the other hand, based on the above discussed potential of Nln substrate peptides in relation to neural repair mechanisms, it is more likely that activity of Nln during recovery period does not have major effect on neural repair mechanisms. This is because, the extracellular peptide substrates of Nln appear to be less uniform in modulating neural repair mechanisms in one direction, i.e.some appear to have potential to enhance/stimulate neural repair, whereas others do the opposite. This is in contrast to the above discussed neuroprotective mechanisms, where the vast majority of neuropeptides inactivated by Nln appear to be neuro/cerebro-toxic in the setting of acute stroke (substance P,bradykinin, neurotensin, angiotensin II, hemopressin), whereas the ones generated by Nln (angiotensin-(1-7), Leu- and Metenkephalins) are neuro/cerebroprotective. This important question on how the function of Nln transitions from acute to chronic phase of stroke, and how it takes part in brain’s highly regulated and complex response to stroke is a subject of ongoing studies in our laboratory.
When it comes to neuroprotection after stroke, it is wellrecognized that most therapeutic approaches in the last two decades have targeted individual pathogenic mechanisms/pathways of the ischemic cascade (Iadecola and Anrather,2011). Unfortunately, such individual approach is not in line with the way that brain protects itself, because the brain relies on coordinated and multifunctional protective mechanisms to minimize stroke injury and recover from it(Iadecola and Anrather, 2011). In this regard, it is important to recognize that Nln, because of its ability to process a number of neuropeptides, could potentially serve as a single pharmacological target to modulate the function of several, independent pathways that are essential in various mechanisms of brain injury or cerebroprotection. A multipathway target like Nln, would be highly desired for stroke pharmacotherapy and would likely be therapeutically more effective in a complex disorder like stroke. If this idea holds true in systematic experimental studies, then small molecule activators of Nln or brain-penetrating variants of Nln, which are in development in our laboratory, could become a new class of drugs for cerebroprotection after stroke. As for any drug target, with Nln too, it will be essential to determine the therapeutic regiments and treatment paradigms that maximize the beneficial effects of Nln, and associated peptides systems, while maintaining the expected side effects at a minimum level. Notably, in addition to determining the pharmacokinetic and pharmacodynamic basis of dosing, these studies should also focus on defining the therapeutic time window and duration of treatment targeting Nln after stroke to properly balance between modulation of cerebroprotective and/or neurorestorative mechanisms by this peptidase.
Acknowledgments:I apologize to colleagues whose work could not be cited because of the limited scope of this manuscript.
Author contributions:The author wrote the manuscript solely and approved the final manuscript.
Conflicts of interest:The author is the senior inventor on a provisional patent, filed by Texas Tech University Systems, focusing on discovery of small molecule activators of peptidase neurolysin.
Financial support:This work was partly supported by research grants from the American Heart Association (14BGIA20380826) and National Institutes of Health (1R01NS106879).
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Kim CY, Seoul National University, Korea.
Additional file:Open peer review report 1.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Oxidative stress battles neuronal Bcl-xL in a fight to the death
- Development and postnatal neurogenesis in the retina:a comparison between altricial and precocial bird species
- Cathepsins in neuronal plasticity
- Cognitive impairment in multiple sclerosis: lessons from cerebrospinal fluid biomarkers
- Progenies of NG2 glia: what do we learn from transgenic mouse models ?
- The NLRP3 inflammasome: a potential therapeutic target for traumatic brain injury