Synthesized graphene oxide and fumed aerosil 380 dispersion stability and characterization with partially hydrolyzed polyacrylamide
Najeebullah Lashari,Tarek Ganat
1 Department of Petroleum Engineering,Universiti Teknologi PETRONAS,32610 Seri Iskandar,Perak Darul Ridzuan,Malaysia
2 Department of Petroleum and Gas Engineering,Dawood University of Engineering & Technology,M.A Jinnah Road,Karachi 74800,Pakistan
Keywords: Aggregation Composites Chemical enhance oil recovery Hydrocarbons Nanoparticles Polymer
ABSTRACT Hydrolyzed polyacrylamide(HPAM)is a commonly used polymer for the chemicals,mining and refining processes of hydrocarbon but suffers from a persistent high-temperature instability problem.In contrast,the nanoparticle suspension remains a technical challenge because of the strong interactions of van der Waal forces within nanoparticles,which always encourage aggregation.This research sought to improve nanoparticles(NP)stability and polymer(HPAM)rheological properties to improved hydrocarbon recovery by utilizing synthesized graphene oxide(GO) nanosheets and fumed Aerosil 380 Silica oxide (SiO2).The aqueous nanocomposites based on HPAM-GO and HPAM-SiO2 in aqueous polymeric solutions have been developed,and its viscoelastic and static behaviour is studied.The results imply that by adding fumed silica NP,the viscoelastic behaviour of HPAM is marginally improved,particularly in high temperatures and salinity,however,the inclusion of GO’s significantly improves the viscosity and stability of the base polymer fluid at high temperatures.The Fourier data for the transformation of the infrared spectrum confirmed that the hydrogen bonding formed between HPAM carbonyl groups and silica NP surface silanol functionality and covalent interlinking of electrostatic h-bonding between HPAM and functional GO contributed to the improved stabilization and improved rheological performance that helps to recover high salinity and temperature hydrocarbons.
1.Introduction
The discovery of new hydrocarbon(HCS)is deemed challenging and present oil and gas findings are merely marginal.Furthermore,the fluctuating oil price in the market has exaggerated the doubts of investors in exploring new developments leaving a vast number of recoverable HCS in diverse mature fields[1].The HCS production is categorized by three different mechanisms,primary,secondary,and tertiary recovery.In first,the production of HCS is recovered through natural drives,whereas in secondary methods various fluids are injected in the reservoir to maintain the HCS recovery.In tertiary recovery,known as enhanced oil recovery (EOR),several techniques (chemical,thermal,etc.) are implemented to recover the remaining oil in place(Fig.1).Chemical enhanced oil recovery CEOR is a very common approach for increasing the volume of HCS recovered,and the most common technique for CEOR use is polymer flooding (PF).This process reduces the mobility of the water phase and therefore improves the efficiency of the sweeping effect [2].
Flooding of the polymer these days,get prior attention at the laboratory and field test levels.The factors limiting the use of polymers in CEOR are changes in rheological behaviour,thermal,and chemical degradation at high salinity.The most commonly used inorganic polymer HPAM (partially hydrolyzed polymer acrylamide) is also no exception; at reservoir temperatures above 70 °C and due to the presence of monovalent and divalent ions,the viscosity of this polymer is also drastically reduced [3–5].In order to address these drawbacks,different approaches have been proposed,such as chemical alteration of polymers,alternatives to catalysts used in polymer alteration to improve the performance of polymers.In recent studies,different nanoparticles (NP) have been used to associate with polymers chemically as well as physically to improve the performance of polymers [6–9].Recent progress in the field of nanotechnology has developed better and more advanced materials that are more effective and eco-friendly.Nanotechnology is a branch of applied engineering that works at the atomic and molecular level (few 100’s nm or smaller) [10].The inclusion of NP forms a colloidally suspended fluid with specific characteristics and alters the behaviour of the base fluid [11].

Fig.1.HCS recovery processes and classification of the improved techniques for oil recovery.
Due to the strong van der Waals interactions between nanoparticles that always support aggregate formation,a homogeneous suspension is still a technical challenge.Some approaches,including physical or chemical processing,are required for stabilizing nanofluids.That involve introducing the polymer,modifying the surface properties of the suspended particles or using strong forces(probe sonication) on nanoparticles clustered together [12,13].If not,aggregation,accumulation and sedimentation may occur and trigger deteriorating suspension properties,such as thermal conductivity,viscosity and increased specific heat.
Nanofluid motion (random flow path) is caused by Brownian motion; the main impact on the performance of NP is attributable to their instability as these NP act differently when the fluid system changes,such as monovalent and divalent charges,tend to attract opposite charges,The temperature increase will result in coagulation and aggregation of NP [14,15].NP also become unstable when their volume is time-dependent[16].The stabilization of NP in the reservoir is based on different characteristics such as particle size,ionic charges present in the porous media,and hydrophilicity of the base solution [17,18].NP themselves have surface charges which are responsible for stability alteration along with van der Waals forces,which also induce intramolecular interaction and eventually affects the stability of NP [10].According to Moonet al.,NP stability is the aggregate of both attractive and repulsive forces.If these repulsive forces (van der Waal forces)are stronger than NP,they remain stable without any aggregation and coagulation [19].The zeta potential value is a dimensionless number that determines the stability of the NP by measuring the negative charge on the surface of the particle correlated with the ionization of different functional groups.Typically,zeta values at or above ±30 mV are determined to be stable due to their electrostatic repulsive forces,concluding that zeta values and NP charge density define their stability [20].
Research studies in the last decade have shown that NP are useful and effective CEOR agents[21–24].The efficiency of aluminium oxide and silica oxide was analyzed and used as a CEOR agent to assess the HCS recovery factor.Through the dispersion of these NP,the total HCS recovery ranged from 62% to 81%,and the main mechanism behind the additional oil recovery was to reduce the interfacial tension and improve the injection fluid viscosity [25].
Graphene has been the focus of many researchers,owing to its remarkable thermal and mechanical properties and its high surface area,among other materials [26,27].As carefully integrated,these single-layer atomic thin carbon sheets will strengthen the host polymers properties at even small concentrations.Owing to Sp2carbon configuration in hexagonal systems,the graphitic carbon NP are part of similar groups.Graphene oxide (GO) is an organic form of almost two-dimensional layers which comprises hydroxyl,carboxyl,-COOH (carboxylic),-C=O (carbonyl),-OH (hydroxyl),and epoxy groups [28].Such functional groups help GO to absorb sex molecules easily,and polymers render their physicochemical interaction a complex material that enhances the material’s properties.GO is,in essence,hydrophilic and has excellent water resilience.The GO is used as a surface-active agent instead of a surfactant that can minimize oil–water (O/W) IFT interaction and communicate with the rock,thereby altering its wettability [29].According to research conducted by Maghziet al.,the addition of NP to a polymer may alter the viscosity during polymer floods,resulting in improved sweeping efficiency [8].
The use of NP to improve the recovery of oil relies mainly on the dispersibility and stability of NP,which are affected by various parameters,such as NP concentration,NP interaction with base fluid,salinity [30].The objective of this study is to investigate the stability and dispersion of GO and SiO2as a composite nanofiller in the HPAM.GO is unique due to its different characteristics,such as high mechanical strength,thermal capacity,electrical properties,and the ability to form a self-associated composite network when used as a crosslinker component [31–33].The silica oxide in fumed form is highly dispersible in hydrophilic solvent due to its high surface area and interaction of silanol groups with hydroxyl groups of polymer makes its highly desirable candidate in developing polymeric nanocomposite.The use of GO and SiO2as a CEOR agent when added to HPAM results in better stability at high temperature and high salinity (HTHS) conditions as no work is done,reported in comparing HPAM with SiO2and GO at HTHS conditions.
2.Experimental
2.1.Materials
The polymer Flopaam 3430S was purchased from SNF SA France,the density of the polymer is 0.8 g·cm-3,and the molecular weight (MW) of 12 MDa.SIGMA-ALDRICH USA provided the salts(sodium chloride and calcium chloride)with more than 99%purity to make electrolyte solution.All chemical reagents,including hydrogen peroxide (HO),Nitric acid (HNO3),Sulphuric acid(H2SO4) H2O2,Potassium permanganate (KMnO4) were obtained from Sigma Aldrich (Merck).Natural graphite flakes were also obtained from Sigma Aldrich (Merck).Fumed SiO2Aerosil?380 has been purchased from Evonik Corporation USA.All other chemicals have been of analytical grade and are used as received unless otherwise stated.
In this research,graphene nanosheets with oxidized groups were synthesized using pure graphene powder according to the modified Hummers technique,as shown in Fig.2 [34].The stepwise procedure is as follows: in 500 ml of a volumetric flask,45 ml of concentrated (H2SO4) and 1 g of graphite flakes were mixed and kept under for 4 h in continuous stirring,and 6 g of KMnO4were gradually added to the prepared solution by controlling the temperature below 15 °C during this process.Slowly,the solution was diluted by adding 90 ml of deionized (DI) water and allowing the solution to blend until a brownish solution has been formed.In an ice bath,the solution oxidation reaction was terminated by treating with 20 ml of H2O2to control the temperature and allowed to stir overnight.The solution was kept for 2–3 h without stirring,and the Whatman filter paper filtered the remaining suspension.The GO formed was washed repeatedly with HCl and DI water to remove residual electrolytes.Finally,the suspended GO was exfoliated with sonication and dried at 50 °C for 8 h to yield GO.
2.2.Samples preparation
The polymer stock solution was prepared by gradually and uniformly adding HPAM powder on the shoulder vortex of DIW,and after homogenization,at 70 r·min-1of vortex whirling motion,a magnetic stirrer is used to swirl the solution steadily for 24 h.(MSH-20D Wise Stir Laboratory Instruments).The stock solution of polymer was kept overnight to hydrate completely and was correctly sealed to avoid chemical degradation due to oxygen.The two-brine solutions were prepared by adding SB1(5% NaCl,mass fraction based),and SB2(8 % NaCl,0.1 % CaCl2) in distilled water.Different concentrations of GO and SiO2(0.01% and 0.1 %)NP allowed to disperse in deionized water and ultrasonically distributed for 30 min in an ultrasonic bath(FB15057,Fisher Scientific)to get stable dispersions.The polymeric nanofluid composites were prepared by adding GO and SiO2solutions with a prepared polymer solution and allowed to mix by continually stirring for 24 h to give enough time for NP to interact with graft polymer in order to get a stable composite.Table 1 displays the important characteristics of the materials used in this study.
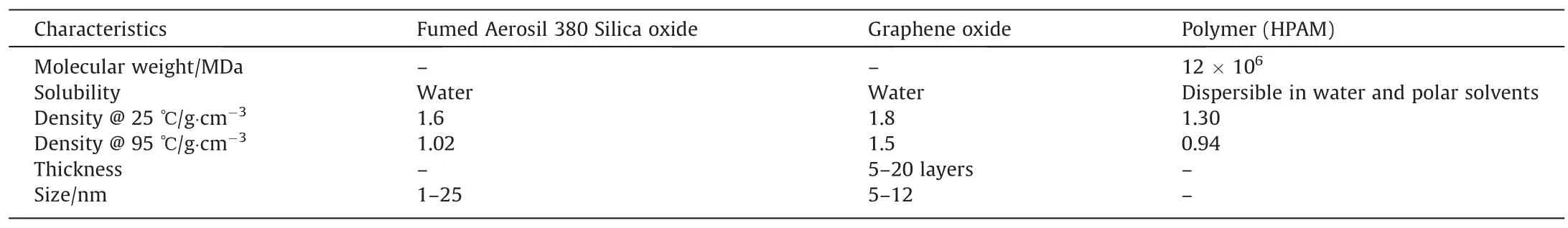
Table 1Nanoparticles and polymer properties used in the study
The prepared synthetic brine solution was then mixed with the individual solutions at room temperature for 24 h to observe the impact of electrolytes on the prepared composite,and samples were placed at 95 °C for one week to check the effect of NP interaction at HTHS conditions.
2.3.Polymer critical micelle concentration
When the concentration of polymer increases,the molecules with solution,begin to self-associate and form coiled like structure which in result reduce the hydrodynamic radius of polymer chains[35].There is a critical concentration where intramolecular starts to self-associate,known as critical micelle concentration (C*)[36].TheC* can be calculated by different methods,like change in fluorescent characteristics when molecular overlapping takes place,change in rheological behaviour,and determining dynamic light scattering[37].In our experiments,the rheological behaviour of HPAM at 30 °C and 95 °C was studied to obtainC* at different temperatures.The HPAM of different MW was analyzed at different concentrations and temperatures.
Stock solutions of different HPAM concentration (%) were prepared using the Equation.
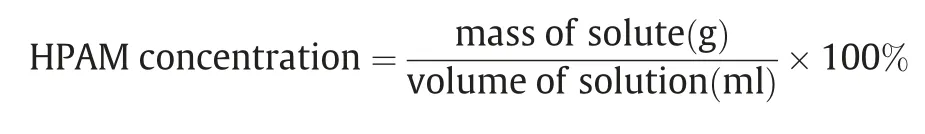
2.4.Dispersion stability analysis
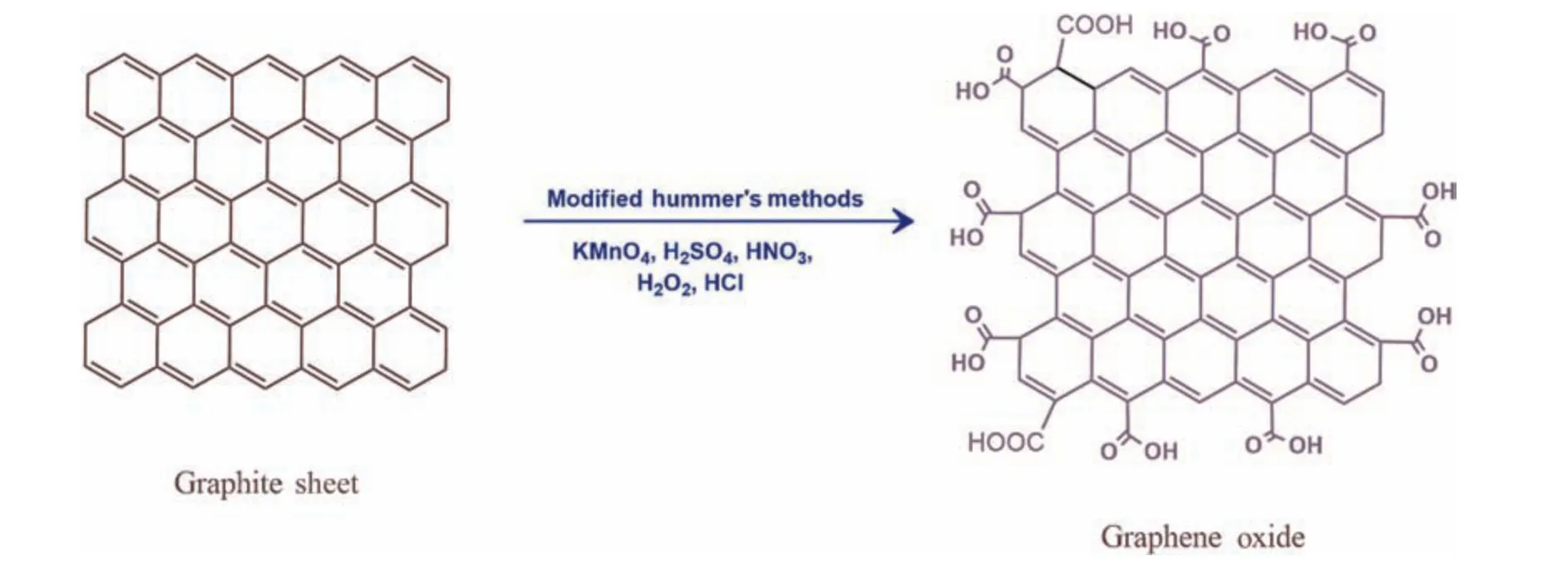
Fig.2.Synthesis of GO by modified hummer’s method.
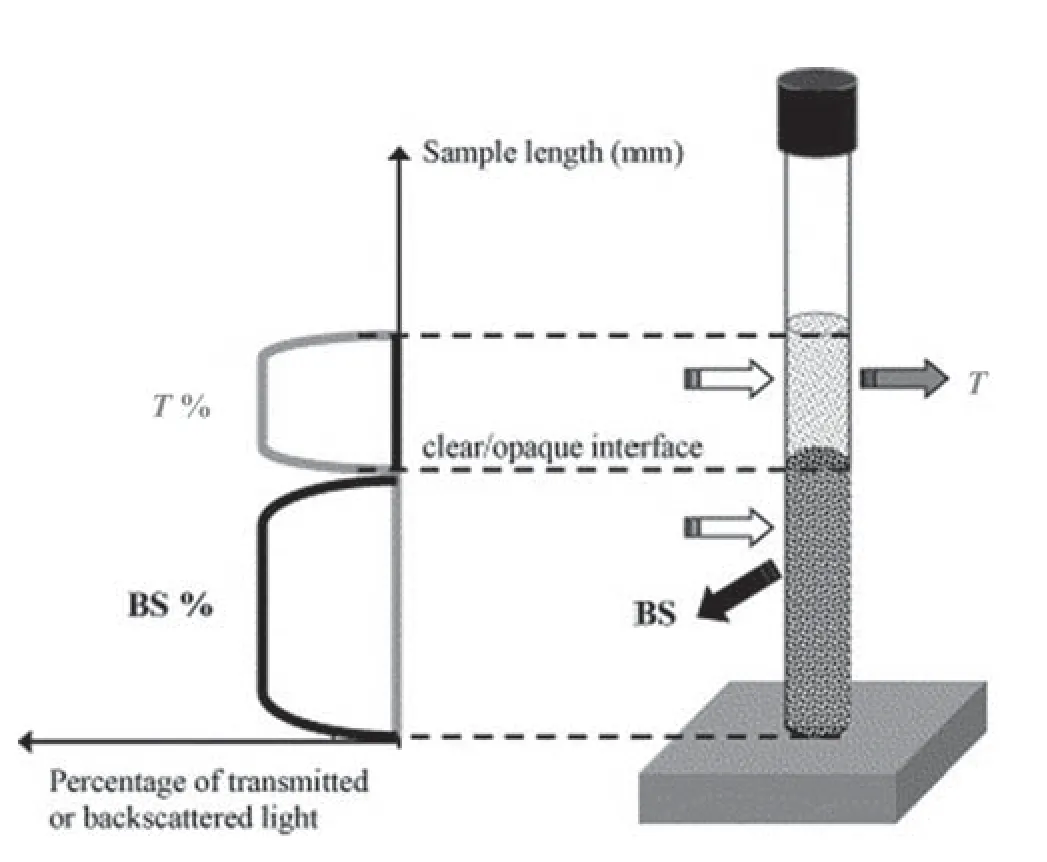
Fig.3.Working principle of TurbiScan.Two detectors of light rays: a transmission(ΔT) and backscattering (BS) [40].

Fig.4.A criterion for polymer critical overlap concentration.
The stability of the GO/HPAM and SiO2/HPAM dispersions has been analyzed using TurbiScan Classic.TurbiScan is used for the analysis of particle migration (creaming and sedimentation) and particle size changes (flocculation and agglomeration) of NP in the solution,as shown in Fig.3 [38].The Turbiscan head consists of a vibrator near the infrared light source (λ=850 nm) and two coincident indicators,a transmission detector that allows the light to pass through the sample (at 0° from the incident beam) and a backscatter detector that receives the backscattered light from the sample (at 135° from the incident beam).The detection head examined the transmission data every 50 μm and every 5 min over 2 h throughout the length of the sample.Variations in the transmission signal (ΔT) were configured at a given time.TurbiScan scans at different pre-programmed times and overlays the profiles on one graph to show the destabilization.The principle on which TurbiScan operates is more favourable compared to other methods,such as dispersions,which can be studied in their original state(under actual storage conditions).Besides,the results are measured without any additional forces.The transmission sensor with a vertical scanner over the sample height at a resolution of 40 μm detects a change in the concentration of NP and its physical heterogeneity [39].
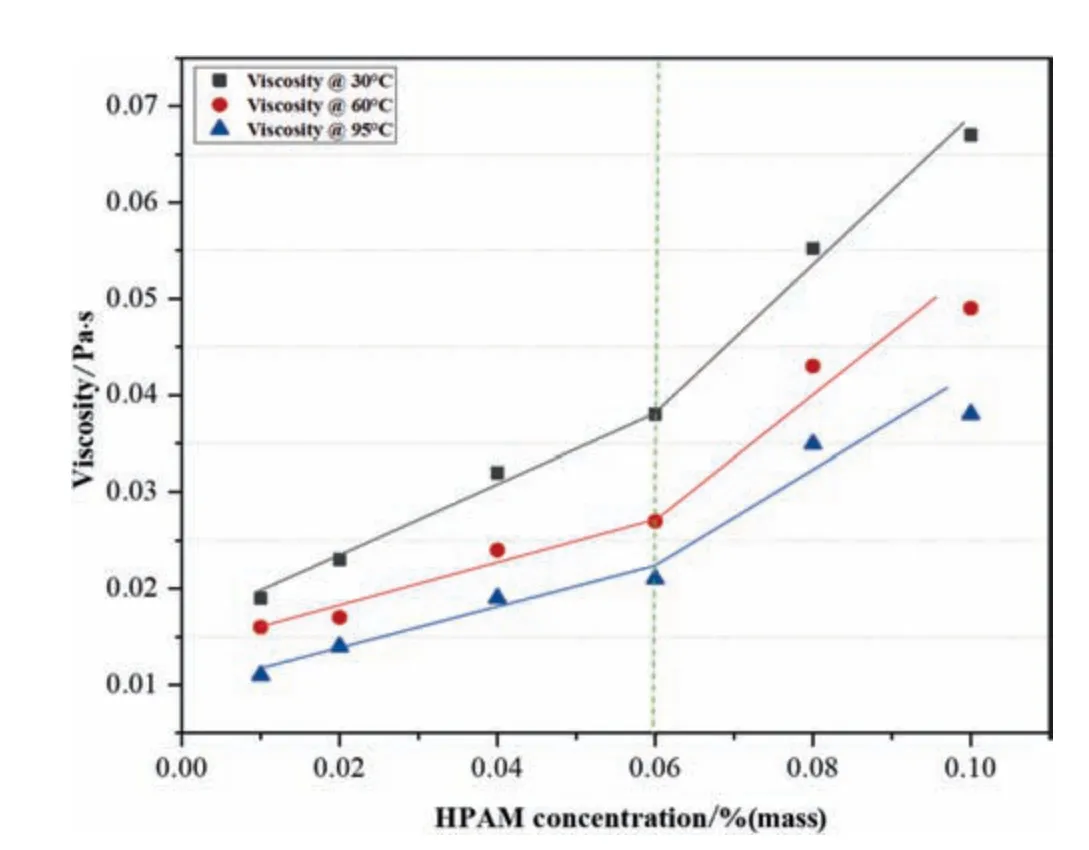
Fig.5.Viscosity of 8×106 HPAM concentration (30,60 and 95 °C,reservoir shear rate 7.3 s-1).
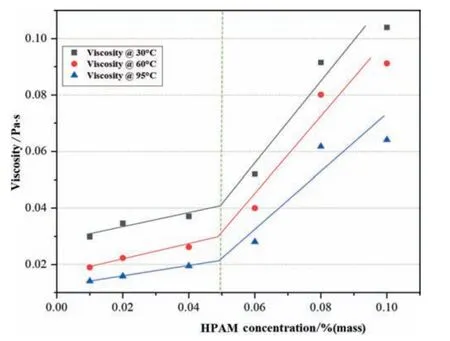
Fig.6.Viscosity of 12×106 HPAM concentration (30,60 and 95 °C,reservoir shear rate 7.3 s-1).
The stability of NP in the HPAM solution in the presence of brine was tested by obtaining zeta potential values.The NP suspension in the fluid is stabilized against sedimentation aggregation depending on the surface charge present on NP.The charge of the particle surface absorbs counter ions in the fluids and creates an ionic layer around the molecule.Zeta potential is considered the balance between the surface of the particle and accompanying the liquid surroundings [41].When this potential is almost nil,there is no charge on this particle,and the solution is likely to aggregate and accumulate.Zeta potentials are essential,therefore,to understand the colloidal stability of suspended particles in liquids.The zeta potential of GO and SiO2at different concentrations in saline solution and HPAM was measured with Particle Sizer (DT1202) instruments using a zeta probe at 25 °C.
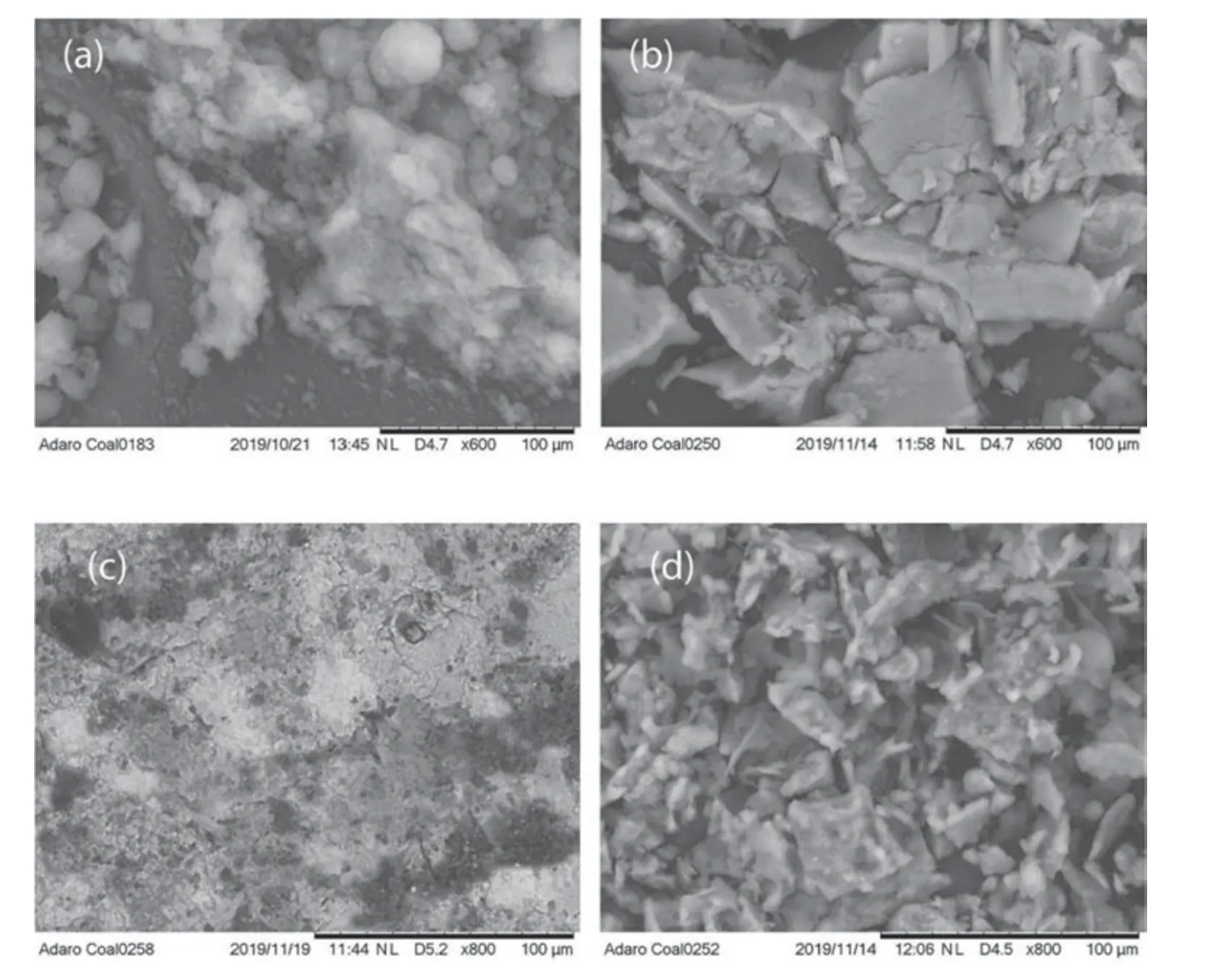
Fig.7.The morphological structure of (a) Pure fumed Aerosil 380 Silica oxide (b) Interaction of fumed Aerosil 380 Silica oxide and HPAM (c) Pure graphene oxide (d)Interaction of graphene oxide and HPAM.
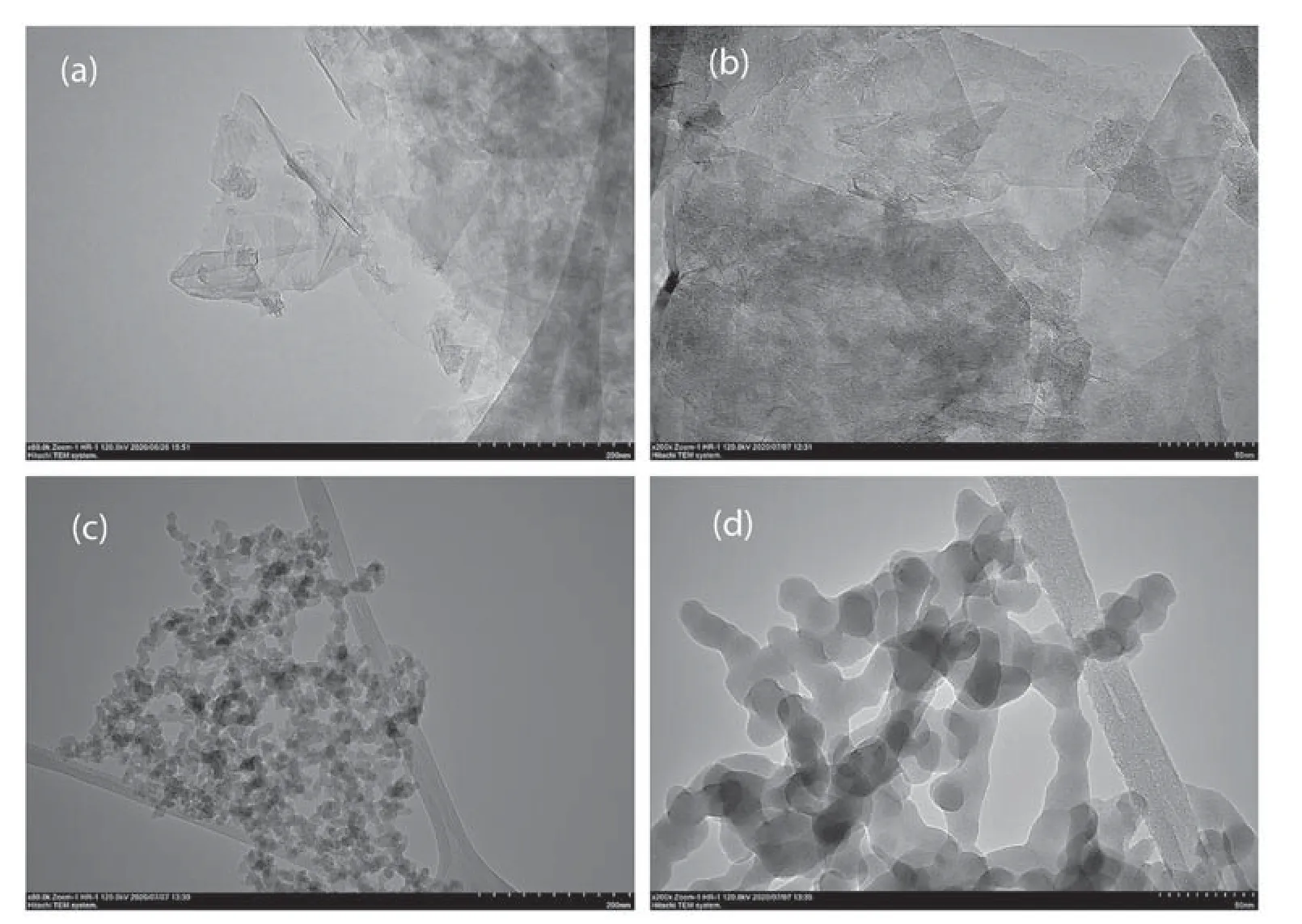
Fig.8.The size distribution of(a)Synthesized GO@200 nm(b)Synthesized GO@50 nm(c)Fumed Aerosil 380 silica oxide@200 nm(d)Fumed Aerosil 380 Silica oxide@50 nm.
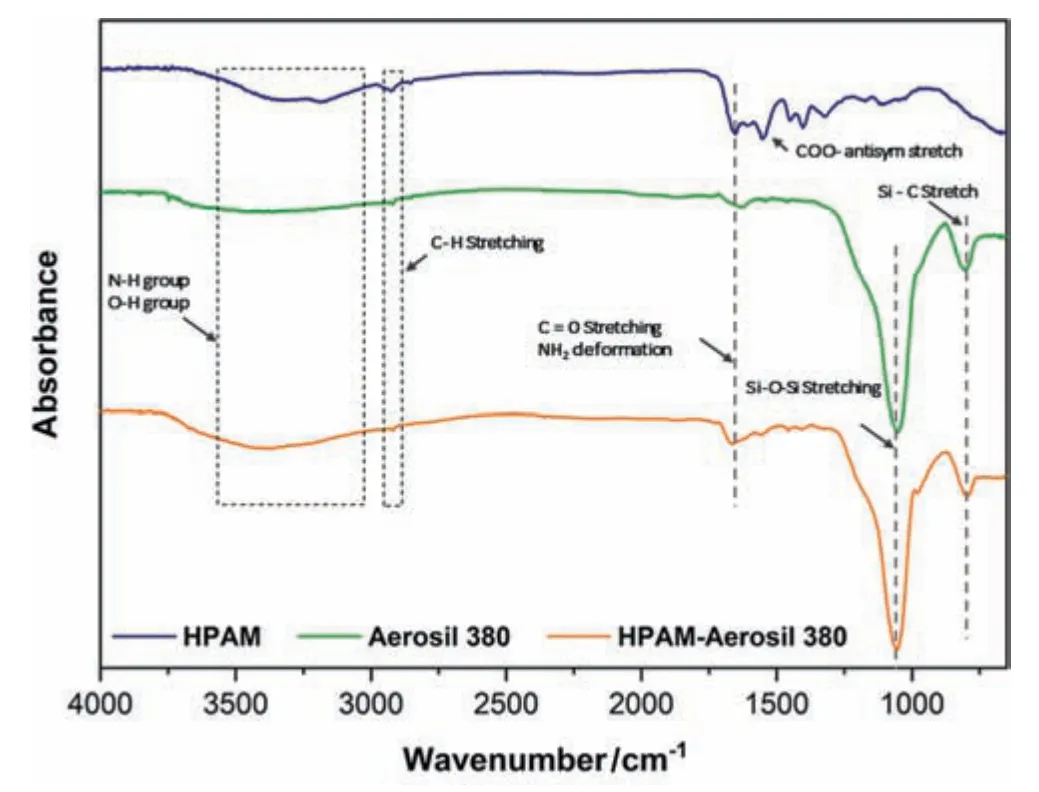
Fig.9.IR spectroscopy of HPAM,Aerosil 380 Silica oxide,and HPAM/Aerosil 380 Silica oxide composites.

Fig.10.IR spectroscopy of HPAM,GO,and HPAM/GO composites.
2.5.Characterization and thermal stability test
The infrared spectrums of sole GO,SiO2,and their interaction with HPAM composites were analyzed by using the Thermo Scientific Nicolet iS10 FT-IR spectrometer.The spectral resolution ranging in from 4000 to 400 cm-1was used to collect data in a 4 cm-1spectral resolution.The morphology of the original HPAM structure and interaction of NP with polymer-forming polymeric nanofluid was attained by using a scanning electron microscope(Tabletop Microscope TM3030,Hitachi).The sole HPAM,NP,and composite were imaged through SEM (Scanning Electron Microscope).The formulated solutions were dissolved in brine solution and preheated at 90 °C.samples were dried and scanned under 15 kV voltage.The thermogravimetric analysis of HPAM,NP,and polymeric nanofluid was measured to evaluate the thermal stability.TGA,Model Q.50,TA Instruments(Thermal Analysis)was used to get thermogravimetric analysis of prepared fluids,for accuracy of results,the fluid was concentrated at 100 times ratio and heated under an N2environment,at a temperature ranging in 25–200 °C.
2.6.Rheological behavior of developed nano polymer composite
A TA discovery hybrid rheometer was used for shear rheological measurements with the geometry of cone and plate.It had a gap of 0.052 mm as normal.Dynamic and steady calculation to examine the rheological activities of suspension of various concentrations was observed.For temperature regulation,the sophisticated Peltier system has been used.A plastic ring solvent trap was used to cover the measuring solution in order to reduce water evaporation during measurements.DI water and oil of proven viscosity at varying temperature have been used to calibrate the configuration of a rheometer.Polymer solutions and HPAM/NP were measured at 30 °C,45 °C,60 °C,70 °C and 80 °C with viscosities at reservoir shear rates (7–54 s-1),respectively.
3.Results and Discussion
3.1.Effect of polymer concentration
Polymer interactions are concentration-dependent,which may influence when additional elements are added,such as surfactants,alkaline,and nanomaterials.This association may be apparent at the first break concentration,known as critical overlap concentration (C*) [42].As Fig.4 shows,in which polymer molecules and other substances start to associate,as when concentration is belowC*,polymer chains are free to move to maintain their hydrodynamic radius ultimately increasing solution viscosity,but when this concentration exceedsC* value then the polymer chains start to entangle,and coiled shape structure forms which reduce hydrodynamic radius ultimately reduction in viscosity is noticed.In order to get desired modifications in the morphological aspect of polymer,theC*is essential to be identified as in forming polymeric nanofluid.There are different methods to identifyC* like dynamic light scattering,variability in fluorenes characters during the micellization process,and through variable view apparatus.
Whereas in this work,rheological investigations of polymer HPAM at 30,60 and 95 °C were used to identifyC*.As Figs.5 and 6 illustrate the 7.3 s-1shear viscosityversusHPAM concentration at different MW,and in these figures,the curve for viscosity rise is fragmented into two portions.In Fig.5,the curve at both temperatures 30,60 and 95°C,below 0.06%(mass),HPAM concentration for MW 8×106(Million Daltons),the change in viscosity trend by increasing concentration rises uniformly till 0.06%,above this concentration a significant increment in polymer viscosity is observed.Similarly,it is also seen in Fig.6 at both temperatures;the viscosity trend of HPAM concentration below 0.05 % shows a slight difference in concentration.For a MW of 12×106,the overall viscosity increment trend suggests that 0.05%is theC*of polymer and is used in all experimental work.The polymer chain interaction increased due to polymer concentration resulting in a more significant frictional effect,which altered the composite stability.
3.2.Mechanism of graphene oxide and fumed Aerosil 380 silica oxide better-thickening effect on polymer
The morphological interaction between GO and SiO2with the HPAM to form polymeric composite was analyzed through SEM and compared with sole NP and composite material.
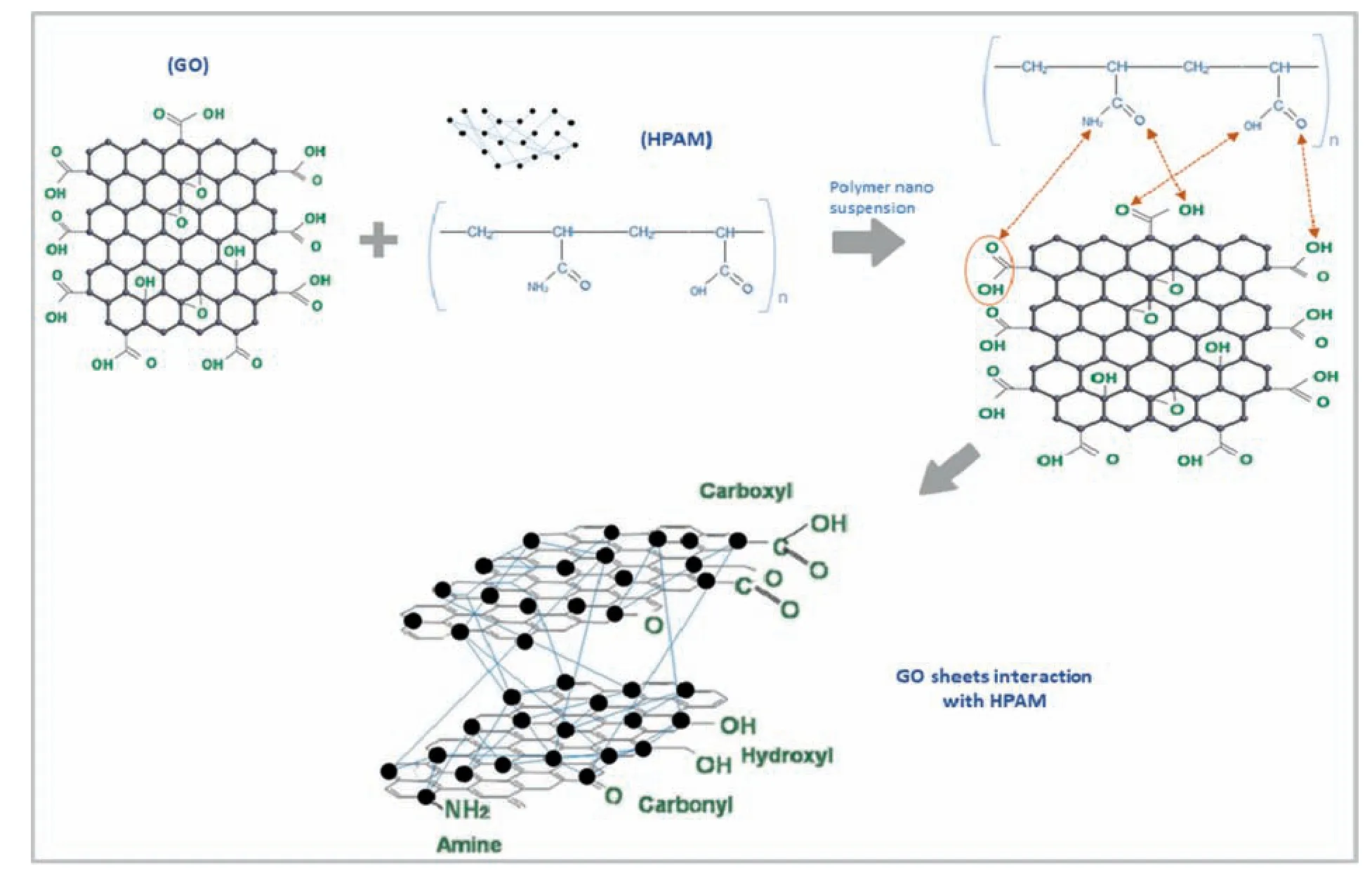
Fig.11.Polymer nano suspension (Interaction of GO function groups with polymer HPAM).
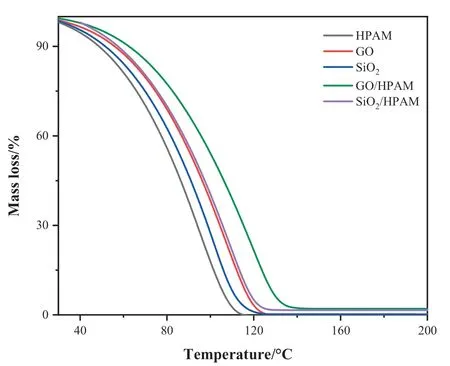
Fig.12.Effect of temperature on HPAM molecules,NP and polymeric nanofluid.
3.2.1.Micromorphology of graphene oxide,fumed Aerosil 380 silica oxide and polymer nanocomposite
Physical analyzes of SiO2,GO,and their impact on the respective HPAM composite were observed by SEM.The freeze-dried sample was used to preserve the structure of the composite network.As in Fig.7(a),the NP-free HPAM spatial structure is observed,the NP structure is scattered,and when added with HPAM,the interaction of NP with amphiphilic polymer is dense.As shown in Fig.7(b),the linkage indicates the stable and dense structure junction in SiO2-HPAM composite.
Similarly,in the synthesized GO Fig.7(c) the exfoliation of GO sheets is observed,and in forming the composite of GO-HPAM,the spatial network interaction is visible Fig.7(d).The associated structure and joining bonds show that the hybrid solution has a stable structure and can pose stability in harsh conditions.
3.2.2.The size distribution of synthesized nanoparticles
TEM is the only direct imaging tool for the detection and calculation of standard aggregate shapes and aggregate sizes.The separated aggregates of high-energy electrons are radiated on the TEM unit.In this scenario,charging impacts are not significant,and the particles can be captured undistorted in their original state.The TEM pictures display traditional wrinkles,and a crumbling GO layout(Fig.8(a)and(b)),Electron diffraction chosen area,as shown in Fig.8(a)reveals that the light and the circles of transparent diffraction are assigned to a GO hexagonal crystal structure.Higher magnification (HRTEM) observations in TEMs indicate that the convergence regions in these aggregates are interacting intensively.Therefore,the main structures have a marksome degree of penetration and the colliding regions are less like a line of pearls with narrow contact points,but more like bottlenecks (Fig.8(c)and (d)).
3.2.3.Characterization of graphene oxide,fumed aerosil 380 silica oxide and polymer nanocomposite
The interaction of NP in HPAM to improve the viscosity of the hybrids is resulted due to the potential bonding effect between NP and HPAM is verified through FTIR(Fourier Transform Infrared)spectroscopy,the two different NP (GO and SiO2) are used as shown in Figs.9 and 10.The peaks in HPAM spectrum 1107,1450,2922 cm-1shows the C-O-C antisym ring structure vibration,COO- sym stretch broad bend in the carboxylic acid group and=C-H bond stretch respectively.
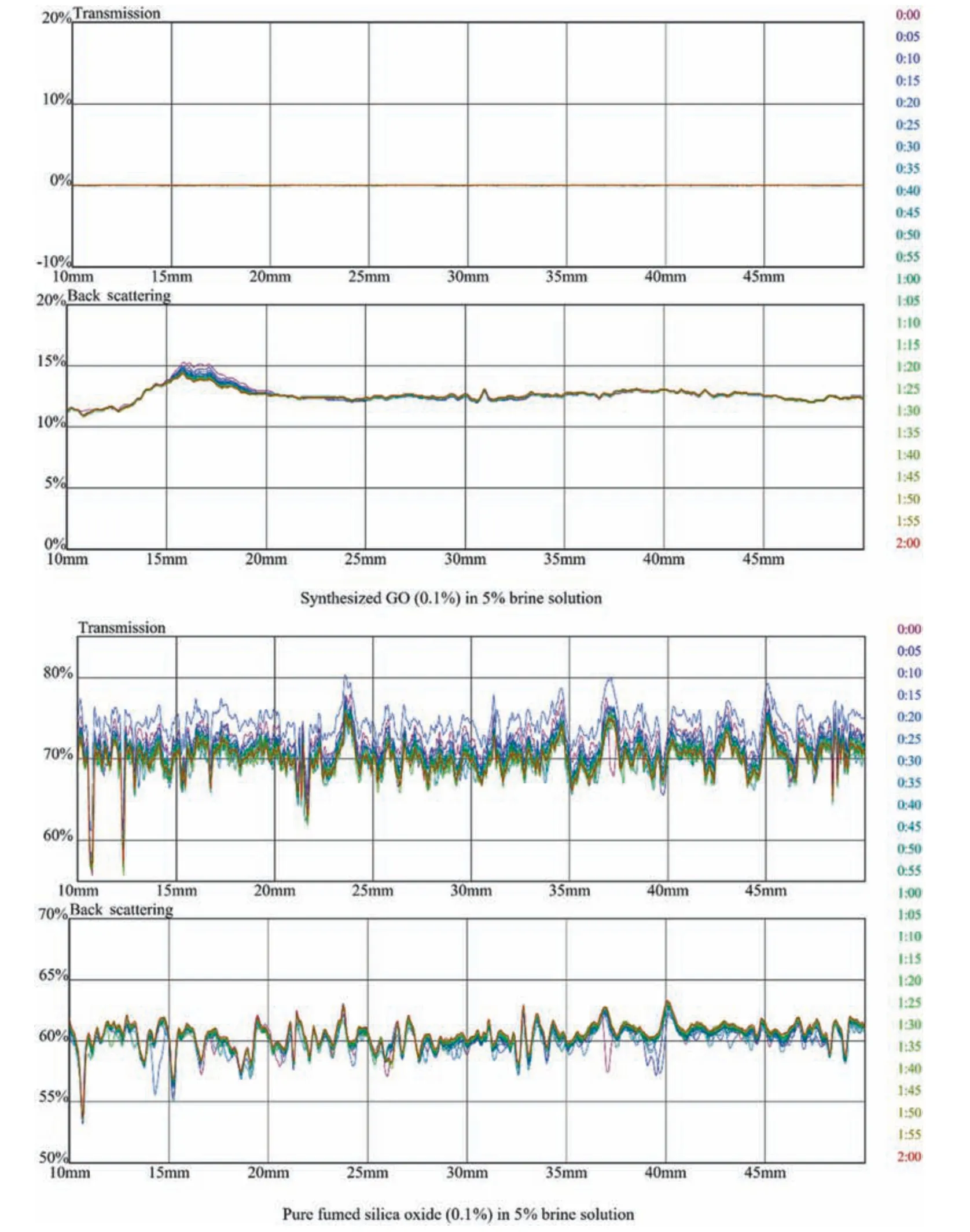
Fig.13.Transmission and backscattering profiles of pure GO and fumed (Aerosil ? 380 SiO2) at 0.1 % in 5 % brine solution.
The amide group chains in HPAM can correspond at 3186 and 2926 cm-1,which shows NH2sym stretch usually in primary amides and distinctive peak at 1654 cm-1represents to the carbonyl group of C=O stretching whereas peak 3389 cm-1is resembled in with secondary amine (N-H) stretch.The Amide I C=O stretch,and Amide II NH2deformation band is recorded at peaks of 1654 cm-1and 1551 cm-1[43].In Fig.9 for SiO2,major peak lies at 1052 and 804 cm-1representing Si-O-Si antisym vibrational stretch and Si-C organosilane stretching.For SiO2/HPAM composite new peaks appear at 1056 cm-1and 796 cm-1on the contrary in pure HPAM; these two peaks are not present.The peak at 1664 cm-1in a hybrid sample is more stretched compared to pure HPAM due to NH2deformation and C=O stretching,the shift effect between SiO2and HPAM might be from covalent bonding crosslinkage [42].The other peak visible at SiO2/HPAM composite at 796 cm-1shows a Si-C stretch much stronger.The C-H stretch peaking at 2918 cm-1indicates H-bonded -OH stretching,which shows carboxylic and amide group interaction with SiO2forming hydrogen bonding between Si surface and nitrogen or oxygen from HPAM.However,in nano polymer hybrid,the peaks in the range of 3150 cm-1to 3853 cm-1become wider in comparison with HPAM due to N-H,Si-C,-OH vibration bending and stretching.The shift in peak from 3182 cm-1to 3397 cm-1is evident of NH2bending to OH stretch,which may be the possible reason in forming a hydrolyzable cross-linking bond between SiO2and HPAM and resulting in improved rheology of a developed composite.
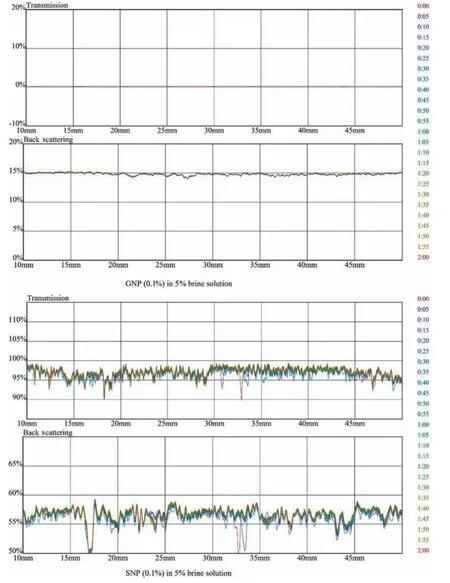
Fig.14.Transmission and backscattering profiles of GNP (Graphene oxide nano polymer) and SNP (Fumed Aerosil 380 Silica oxide nano polymer) of 0.1 % in 5 % brine solution.
In Fig.10 the FTIR spectrum of GO NP represents the broad and clear peaks at 3163,1615,1038 and 679 cm-1referring to OH symmetring and asymmetring stretch vibration[44],the doublet peaks at 2851 and 2910 cm-1indicates antisym and sym stretching of CH,whereas the ranges at 1025 and 1232 cm-1refer to CH-O-H stretching vibration and C-O stretch respectively.The above bonding interactions containing oxygen functional groups validate the synthesis of GO.The IR spectra GO/HPAM composite forms new peaks demonstrating improved viscosity and stability of the hybrid suspension.The new peak at 1614 cm-1states the transformation of GO-COOH into-COO-doublet stretching due to rotational isomerism.The similar peaks visible in HPAM (3289 and 2946 cm-1)were also observed in GO/HPAM hybrid but shifted to lower spectrums(3310 and 2906 cm-1),showing the stability of nanohybrid.The epoxide functional group in GO (CH-O-H) at 1038 cm-1moved to lower bending proving the presence of hydrogen bonds.The new peaks absent in pure HPAM are formed in GO/HPAM hybrid at 1096,and 1521 cm-1shows the C-O-C carbon ring stretch formed in an aromatic compound,which could be the result of the cross-linking of covalent hydrolyzable bonding between GO and HPAM [42,45].The new peak appeared at 2826 cm-1,showing the C-H stretching mode either attached to O or N,confirming the covalent bond formation in between Carbon of GO and hydrogen of the HPAM amide group [46].The N-H stretching in hybrid is noticed at 3281 cm-1formed due to electrostatic interaction between the amide group of polymer and hydrogen in the GO,the potential interaction of GO with HPAM is illustrated in Fig.11.The carbonyl stretching vibration formed at peak 1865 cm-1and two bands formed NH2stretching and C=O band stretch forms N-H bending vibration and C-N deformation,which concludes the interaction of carboxylic group in GO and amide group in HPAM.

Fig.15.Transmission and backscattering profiles of GNP and SNP of 0.1 % in 8 % brine solution.
The change in temperature effect on HPAM,GO,SiO2respective polymeric nanohybrid with 500×10-6of each solution in water was estimated by measuring TGA in N2from room temperature to 200 °C (Fig.12).The major weight loss shown is of water,but the minute amount of HPAM and NP shows the change.The analysis shows that loss of mass gradually drops from 60°C,the HPAM loss compare to NP is more as when it reaches 110°C it completely diminishes but the NP alone shows the loss to around 120 °C but the in nano polymer hybrid after loss of water the amount remains constant in both SiO2/HPAM and GO/HPAM nanocomposites.
3.3.Stability of nanoparticles and polymeric nanofluid
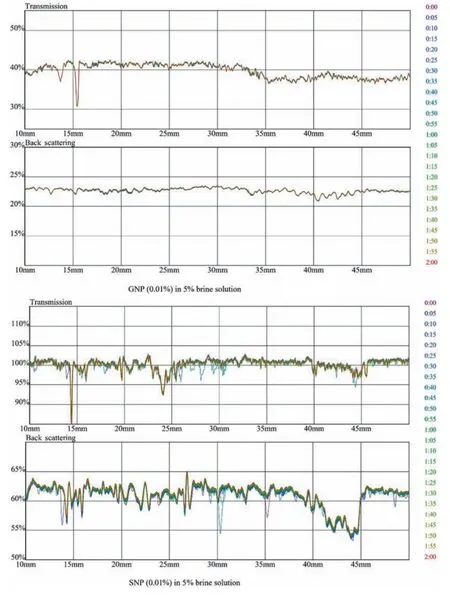
Fig.16.Transmission and backscattering profiles of GNP and SNP 0.01 % in 5 % brine solution.
Usually,HPAM molecules can form a concentration-based association that can be observed when it is allowed to blend with molecules of another element.As discussed above,the intramolecular attraction of polymer hydrodynamic chains plays an important role in the development of polymer nanofluid,in this section,due to theC* of HPAM with MW 1.2×106,a concentration of 0.05%(mass),which is almost belowC*,was selected to analyze the stability impact of SiO2/HPAM and GO/HPAM.
The concentration ranges of GO and SiO2selected for experimentation were 0.01 %,and 0.1 %,each sample was prepared by adding 0.05%HPAM and the brine solution of SB1and SB2to check the effect of electrolyte on developed polymeric nano composite.The samples were kept at 95°C for one week to analyze the HTHS effect on the dispersibility of NP in the polymer.
The most common method to decide nano composition stability is the static position method.In this method,the sample is allowed to stand in a container throughout the duration of observation,and the naked eye observed the distance or colour difference in sedimentation.Still,this technique cannot identify the composite’s true stability.As illustrated in Fig.3,the dispersibility and suspension behaviour of NP in brine and HPAM were analyzed and results recorded for 2 h in an interval of 5 min by using TurbiScan Analyzer,
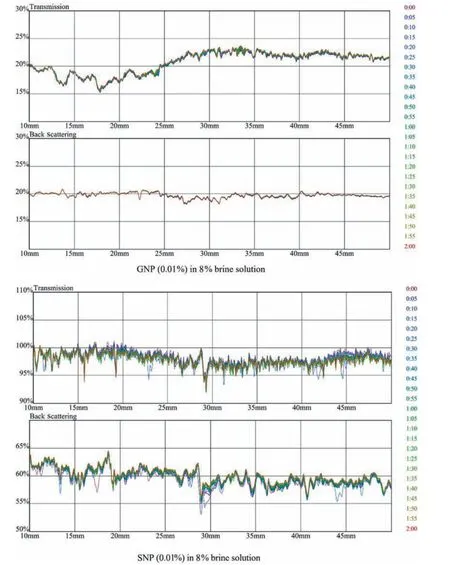
Fig.17.Transmission and backscattering profiles of GNP and SNP 0.01 % in 8 % brine solution.
The transmission and backscatteringversussample height are measured.The transmission and backscattering interpretation explains the variation in profile caused due to sedimentation and change in particle size over the sample height.Within allowed time,the change in transmission and backscattering profile occurs within the sample cell.Howsoever,if the dispersion of particles is stable in defined sample height,then no noticeable change in the profiles is recorded.Pure GO and SiO2with 0.1 % is tracked,as shown in Fig.13,The results focus on transmission and backscattering behaviour through the 40 mm height of sample tube The transmission profile of GO lies at 0 value due to concentration of solution whereas in backscattering profile the initial aggregation occurs due to monovalent and divalent ionic charges on the contrary in SiO2the aggregation and coagulation are occurring throughout the sample height.
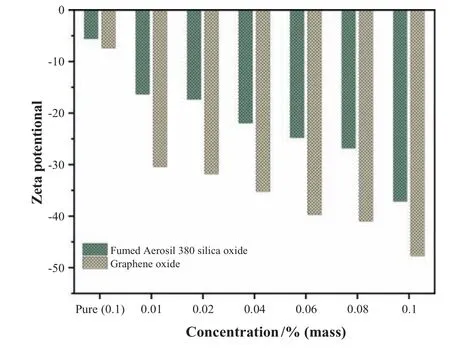
Fig.18.Comparison of zeta potential values of pure GO and SiO2 and in HPAM of concentration 0.05 %.
The transmission profile of GO lies at 0% due to its opaqueness,while in the backscattering profile,the change in particle size is observed whereas pure SiO2shows a shift in particle size in both transmittance and backscattering profiles.The variation in GO profiles compared to fumed Aerosil SiO2is very small,which shows the hydrophilic characteristic of GO in brine water.A representative example of stable suspension (Figs.14 and 16) is the suspensions containing 0.1% and 0.01% of GO and SiO2respectively in HPAM/GO and HPAM/SiO2composites.In Fig.14,the backscattering profile of GO at 15% shows no aggregation and coagulating of NP,whereas in SiO2,transmittance and backscattering profiles show aggregation of NP.The change in salinity from 5%to 8%further affects the stability of NP in polymer; this change is due to increase in ionic charges present in a brine solution which are attacking the amide and carboxylic groups in the polymer as shown in Figs.15 and 17 despite the increase in monovalent and di-valency of ionic charges the GO interaction in polymeric solution is more stable compared to SiO2at both concentration of NP(0.01%and 0.1%).The mechanism of dispersion relies on surface charge and presence of functional groups in a polymer which results in molecular interaction with amide and carboxylic groups present in HPAM.The results indicate that GO is more stable in HPAM composite than fumed Aerosil SiO2as in the height portion of the sample container,no change in particle size or sedimentation occurs throughout all range of GO concentration.
The zeta potential values of pure GO,and SiO2in 5%brine solution showed lower values due to salinity as the electrolytes in solution interact with NP.Due to increasing solvent polarity,the aggregation of NP takes place,but compared to SiO2,the GO remains more stable in the presence of salt.Since salinity tolerance is responsible for limiting the use of polymers in CEOR mechanisms,and because HPAM is a polyelectrolyte,the hydrodynamic chain size is reactive to concentrations of electrolytes.The electrolyte effect on polymeric nanofluid formed by two separate NP is contrasted in this research.The results in Fig.18 shows zeta potential values of polymeric nanofluid to varying concentrations of NP and 0.05 % HPAM.The composites zeta potential demonstrates strong cross-linkages of HPAM and GO nanosheets concerning to SiO2,indicating better dispersion stability.
3.4.Rheological analysis of polymeric nanofluid
3.4.1.The HPAM solution’s molecular interaction with nanoparticles in the presence of electrolyte

Fig.19.(a) Interaction of polymer with electrolytes (b) nanoparticles and polymer interaction in presence of electrolytes.

Fig.20.Effect of NP concentration and electrolyte on graphene oxide nano polymeric(GNP)composite(a)0.01%GNP in SB1@different temperature ranges(b)0.01%GNP in SB2 @ different temperature ranges (c) 0.1 % GNP in SB1 @ different temperature ranges (d) 0.1 % GNP in SB2 @ different temperature ranges.

Fig.21.Effect of NP concentration and electrolyte on silica nano polymeric composite(a)0.01%SNP in SB1@different temperature ranges(b)0.01%SNP in SB2@different temperature ranges (c) 0.1 % SNP in SB1 @ different temperature ranges (b) 0.1 % SNP in SB2 @ different temperature ranges.
The HPAM molecular normally exhibits a concentrated association of polymer chains,as illustrated in Fig.19(a).The polymer backbone chain contains carbon molecules in the form of flocks and lumps,under low saline conditions these chains are separated in a long and straight network but in high salinity,the chains start to the coil and reduce the volume of polymer concentration.The polymer chain contains carboxylic and amide groups in a structure which are prone to react with monovalent and divalent ions present in brine.The polymeric chain network shielded through carboxylic and amide functional groups when reacted with (Na+,Ca2+) leads in decreasing hydrodynamic volume of the polymer,resulting in a decrease in viscosity (Fig.20 and 21).Polymeric molecules with high salinity salts are close to the cotton-like structure which have a strong propensity to develop bonding effect with mono-and divalent ion,contributing in decreased viscosity.Due to the existence of calcium ions,the difference in polymer viscosity which possesses divalent ions has been shown to reflect an increased viscosity loss compared to a monovalent ion.The viscosity loss in HPAM solutions may arise due to the deformation of molecules into spherical and non-ionic molecules [8].The interaction of (Na+,Ca2+) with HPAM is expressed in Fig.19(a),when the polymer is introduced to brine the monovalent and divalent ions present in brine strongly interact with amide and carboxylic groups present on the backbone of a polymer chain,the interaction is dependent on the concentration of ionic charges present,the molecular weight of polymer and degree of hydrolysis at ambient conditions.In Fig.19(b) the presence of nanoparticle possess hindrance in direct interaction of electrolytes with polymer due to strong OH bonding between NP and polymer,in the presence of silica oxide,the particles are absorbed onto hydrophobic chain due to development of hydrogen bonds between HPAM carbonyl groups and silica silanol groups.In the presence of high concentrated NP,the Si-O groups combine to form Si-O-Si groups which ultimately improves the viscosity of the polymer.Fig.19(b) shows a possible mechanistic diagram of the nanocomposite GO/HPAM.On the edges and basal planes of GO nanosheets were the many functional groups which contained oxygen(carbon,hydroxyl,epoxy groups,etc.).The presence of a -COOH group in the GO can generate HPAMgroup in order to generate the-/-OOC ion pairs after interacting with HPAM.In addition to electrostatic interactions,different forms of hydrogen bonding were also found in the composites,In particular:between carboxyl groups of HPAM and hydroxyl groups of GO,and the other two existed in the centre of the protonHPAM groups,along with epoxy and the carboxyl groups of GO,respectively as shown by FTIR observations.
3.4.2.The effect of rheological behaviour of GNP and SNP hybrid dispersion in brine solutions
The NP/HPAM hybrid dispersions show similar rheological behaviour as HPAM solutions in DI water,better than a brine solution.The incorporation of mono-and divalent ions in HPAM,as stated in Section 3.4,has weakened the solution’s viscosity and temperature effect is independent of brine concentration.The inclusion of GO in HPAM as in Fig.20(a)and(b)at same NP concentration the viscosity reduction from 8 mPa·s to 5 mPa·s and 10 mPa·s to 2 mPa·s is Binding/interaction with ions and oxygen molecules because of cation dipole.Binding forces between cations and amino groups (NH2) were also considerably smaller than the oxygen-cation relationship.Divalent ions such as Ca2+bear a higher charge density of the HPAM molecular structures than monovalent (Na+) ions and create a more solid bond with the amide group than monovalent ions that weaken N-H and C-O but increasing NP concentration to 0.1 % the viscosity of solution ultimately improves as shown in Fig.20(c)and(d).In the presence of electrolyte solution,GO-HPAM hybrid scatters demonstrated an increase in viscosity relative to SiO2-HPAM.The ion–dipole relationship between GO NP and ions (Ca2+and Na+) is more prominent than that between SiO2-HPAM (Fig.21(a) and (c)),respectively.The increase in the concentration of SiO2did not show an evident increase compared to 0.01 % (Fig.21(b) and (d)) which may be due to only silanol group presence whereas in GO the interaction of different functional groups (carbonyl,carboxylic,epoxy)and surface availability weaken the ionic interaction with polymer.Owing to the strong ion–dipole bond between electrolyte cations and oxygen atoms from GO NP,the hybrid dispersibility of GOHPAM has a higher viscosity than SiO2-HPAM hybrid dispersibility.Therefore,in the presence of NP,the number of cations targeting the HPAM molecules has been limited to a certain extent,and chemical deterioration is decreased.FTIR study also defined in detail the approach used to increase the viscosity of HPAM in the existence of NP.
4.Conclusions
This research investigated the incursion of various NP into the polymer in order to examine hybrid composite stability.The use of GO and SiO2as a reinforcement material in the HPAM under HTHS conditions has been successfully demonstrated.The CAC value of the polymer to formulate polymer nanofluid is optimized by analyzing the rheological behaviour of HPAM at ambient and high temperatures,the optimum concentration of 8×106and 12×106for HPAM was 0.06%and 0.05%respectively The attractive force of different functional groups,as discussed in FTIR results,could have enhanced dispersion stability compared to sole NP in the aqueous phase.For NP suspension in HPAM,the profiles produced by TurbiScan have been correlated with the transmission and backscattering.The light transmittance and backscattering were found to be a useful tool to analyze nano-polymer dispersion stability.The SiO2dispersion stability was less in comparison with GO,and when treated with HPAM,the present study found that GO/HPAM composite fluid can be potentially feasible nanocomposite for nano-CEOR.Nano addition in HPAM changed the morphology of composite by replacing the functional groups of the HPAM with NP,and the stability of pure and suspended NP in the HPAM was showed were GO,in comparison to SiO2,showed better suspension at all concentrations.The zeta potential values confirmed the stability of NP as at sole suspension the GO and SiO2values laid below -10 in brine 5 % solution,but when incurred in HPAM,the zeta values went up to-40,which showed high stability of NP in a polymeric nanofluid.The rheological behavior of developed polymeric under harsh conditions effects the viscosity specifically the presence of divalent ionic charges present in brine solution despite high salinity and high temperature the inclusion of GO NP in HPAM improves the rheological characteristics of developed polymeric nanofluid.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project is supported by Dawood University of Engineering and Technology Karachi,Pakistan,under the project: ‘‘Strengthening of DUET.”
 Chinese Journal of Chemical Engineering2021年6期
Chinese Journal of Chemical Engineering2021年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes
- Functional monodisperse microspheres fabricated by solvothermal precipitation co-polymerization
- Synthesis and characterization of caprolactone based polyurethane with degradable and antifouling performance
- Removal of lead (Pb(II)) and zinc (Zn(II)) from aqueous solution using coal fly ash (CFA) as a dual-sites adsorbent
- Catalytic performance improvement of volatile organic compounds oxidation over MnOx and GdMnO3 composite oxides from spent lithium-ion batteries:Effect of acid treatment
- Application of fracturing technology to increase gas production in low-permeability hydrate reservoir:A numerical study
