Functional monodisperse microspheres fabricated by solvothermal precipitation co-polymerization
Fenghao Guo,Yuanyuan Ding,Yanyan Wang,Xiao Gao,Zhiyong Chen
Shandong Provincial Key Laboratory of Fluorine Chemistry and Chemical Materials,School of Chemistry and Chemical Engineering,University of Jinan,Jinan 250022,China
Keywords: Monodisperse microsphere Solvothermal Methacrylic monomer Precipitation polymerization Core-shell structure
ABSTRACT Simultaneous achievement in high solid content and high microsphere yield is deemed a challenge in the fabrication of monodisperse microspheres by precipitation polymerization.We herein demonstrate that micro-sized monodisperse poly(methacrylic monomer-divinylbenzene) microspheres containing epoxy,lauyl,carboxyl and hydroxyl functions can be fabricated by solvothermal precipitation copolymerization at 20% (mass) monomer loading with over 94% microsphere yield.The morphology and porosity of the obtained particles can be readily tuned by cosolvent-acetonitrile binary solvents.Addition of a small amount of cosolvent that has similar solubility parameter to that of the functional monomer can significantly improve the monodispersity of the obtained microspheres.When tetrahydrofuran was used as the co-solvent,the surface area of the highly porous microspheres achieved higher than 400 m2·g-1.Solvothermal precipitation co-polymerization can be expected in scale-up fabrication of various monodisperse functional microspheres free of any surfactant and additive.
1.Introduction
Precipitation polymerization has received growing attention in recent years,as it is a unique way to produce monodisperse microspheres without any surfactant and additive among state-of-theart polymerization techniques [1–3].Since thermal precipitation polymerization was developed by St?ver group[4,5],several modified approaches have been exploited,including distillation precipitation polymerization [6,7],photo-induced precipitation polymerization [8–10],controlled living precipitation polymerization [11,12],reflux precipitation polymerization [13],redoxinitiated precipitation polymerization [14,15]and self-stabilized precipitation polymerization[16],based on radical polymerization mechanism besides step-growth polymerization one [17–19].Diverse monodisperse microspheres by these approaches have been found in numerous applications such as enzyme immobilization,stationary for chromatography,cell imaging,and biosensors,etc.[20–23].
Strategies for fabrication of functional microspheres by precipitation polymerization can be categorized into two groups,i.e.,grafting technique and precipitation co-polymerization.Grafting technique can be subdivided into two-stage precipitation polymerization [24–26]and surface modification [27–29].Li and St?ver first put forward two-stage precipitation polymerization[24].They fabricated highly crosslinked polydivinyl benzene (PDVB) as core particles by precipitation polymerization in the first stage,and then introduced functional monomer,e.g.chloromethylstyrene,and crosslinker in the second stage polymerization,thus obtaining core–shell microspheres.Through two-stage precipitation polymerization,complex core–shell structures,e.g.surface molecularly imprinted polymers,can be designed and applied in solid phase extraction [26].In surface modification,residual double bonds on the core particles can be converted into various functionalities by chemical reaction,especially by click chemistry [30].Recently,Liet al.produced double shelled particles using a combination of distillation precipitation polymerization and thiol-yne reaction[31].A high graft density could be obtained by virtue of the efficient reaction between thiol groups and alkyne groups on the particle surfaces.
Compared to grafting technique,precipitation copolymerization is one-step process.By this process,a variety of functional microspheres can be prepared,including poly(methyl methacrylate-co-divinyl benzene) [5,32],poly(glycidyl methacrylate-co-divinyl benzene) or poly(glycidyl methacrylateco-ethyleneglycol dimethacrylate) [9,33,34],poly(hydroxylethyl methacrylate-co-divinyl benzene) [35],poly(lauryl methacrylatedivinylbenzene)[14],poly(methacrylic acid-co-divinyl benzene)or poly(methacrylic acid-ethyleneglycol dimethacrylate)[36,37],poly(acrylonitril-co-styrene) [15,38],poly(bismaleimide-co-styrene)[39]microspheres,etc.Recently,Schenning and colleagues described a method to obtain monodisperse liquid crystal-based microspheres by precipitation polymerization [40].They found that liquid crystal molecules aligned within the microspheres that could be used as sorbent or cargo for adsorbing or releasing target molecules in aqueous systems.Jiet al.reported that zwitterionic magnetic composites were fabricated by one-step distillation precipitation polymerization [41].The resultant microspheres with zwitterionic-polymer shell were demonstrated in selective enrichment of glycopeptides from real samples.Tang and co-workers combined 4-vinylbenzyl-modified tetraphenylethylene,a vinylmodified aggregation-induced emission (AIE) monomer,styrene and maleic anhydride into a precipitation co-polymerization [42].By this way,the nucleation and particle growth in precipitation polymerization was first be monitored in real time due to the increase in fluorescence intensity of the particles.The aforementioned processes of precipitation polymerization are usually restricted in laboratory scale.The obstacle is attributed mainly to a low monomer loading that is normally lower than 5 % (mass),along with a relatively low yield of microspheres (typically below 50%).
Up to date,only a few studies concern the investigations on high monomer loading in precipitation polymerization.Kong and colleagues recently developed several methods of step-growth precipitation polymerization to produce uniform-sized polymeric microspheres such as polyuria [43]and polyurethane microspheres [44].They found that the functional microspheres could maintain polydispersity index (PDI) below 1.05 even at monomer loading higher than 20 % (mass).In our recent study [45],we put forward a method termed solvothermal precipitation polymerization (SPP) that combined solvothermal process and classical precipitation polymerization.We found that the DVB concentration could be increased up to 20 % in the preparation of monodisperse PDVB microspheres with over 90% microsphere yield.Wang and coworkers first produced monodisperese ethyleneglycol dimethacrylate based microspheres in alcohol-water systems with a high monomer loading and high microsphere yield by SPP[46].In this study,we aimed to use solvothermal precipitation copolymerization for synthesis of monodisperse microspheres containing varied functionalities.Glycidyl methacrylate (GMA),lauryl methacrylate (LMA),methacrylic acid (MAA) and 2-hydroxyethyl methacrylate (HEMA) were chosen as functional monomers to copolymerize with divinylbenzene.We mainly focused on reaction conditions how to affect the morphologies of the functional microspheres at high monomer loading(20%),especially how to improve the microsphere uniformity and porosity through altering solvent compositions.
2.Experimental
2.1.Materials
Glycidyl methacrylate (GMA),lauryl methacrylate (LMA),methacrylic acid (MAA),2-hydroxyethyl methacrylate (HEMA)and divinylbenzene (DVB,80% divinylbenzene isomers) were obtained from Aladdin Chemistry Co.Ltd.(Shanghai,China) and free from inhibitor by passing through basic Al2O3(Sinopharm Chemcial Reagent Co.Ltd.,Shanghai,China) prior to use.2,2-Azobisisobutyronitrile (AIBN) was purchased from Damao Chemical Reagent Co.Ltd.(Tianjin,China)and recrystallized from methanol.Acetonitrile (ACN),toluene,tetrahydrofuran (THF) and propanol were of analytical grade and were used without further purification.Deionized water was produced by a Millipore water system composed of Milli-RO 60 and Milli-Q SP.
2.2.Polymerization
Polymer functional microspheres were prepared by solvothermal precipitation co-polymerization according to our previous description with a slight modification [45].Typically,functional monomer (6.07 mmol),DVB (24.27 mmol) and AIBN (8 % (mass)relative to monomers) were dissolved in acetonitrile or the mixture of acetonitrile and co-solvent.The weight of the total monomers corresponded to 20 % with respective to the solvent.The pre-polymerization mixture was degassed by bubbling dried nitrogen for 15 min and then sealed in a teflonlined stainless-steel autoclave (50 ml capacity).The autoclave was heated from ambient to 85°C within 30 min and maintained at 85°C for 4 h without shaking or stirring during the whole polymerization,and then cooled down to room temperature.The resultant particles were separated by vacuum filtration over a G-5 sintered glass filter and washed by methanol for three times.All the obtained polymer particles were dried overnight under vacuum till constant weight.
2.3.Characterization
The number-average particle size(Dn)and polydispersity index(PDI) of the resultant polymer particles were determined by scanning electron microscopy (SEM,QUANTAFDG 250,FEI,USA).DnandDw(the weight-average diameter) were achieved using software of NANO MEASURER by counting at least 100 particles from the SEM pictures according to the equations here below:
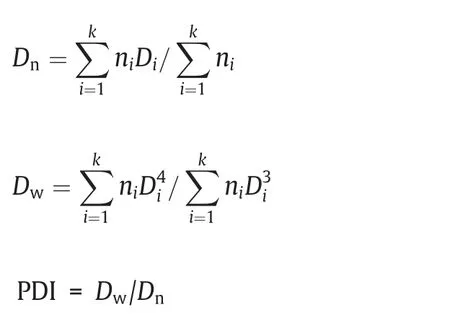
whereniis the number of the measured particles,andDiis the particle diameter of determined microspheres,respectively.The yields of the microspheres were determined gravimetrically.
Transmission electron microscopy (TEM) tests were conducted on a FEI Talos F200X at an accelerating voltage of 200 kV.
The swelling test was carried out by immersing 1 % (mass) of the sample in 20 ml of THF and then ultrasonicated for 10 min,followed by incubating the sample in a water bath with the temperature set at 50 °C for 12 h [33].The particles were collected by centrifugation and repeatedly washed with water and methanol,followed by drying under a vacuum at ambient temperature.
FT-IR (Fourier transform infrared spectroscopy,PerkinElmer Spectrum one,PerkinElmer,USA) analysis was used to verify the characteristic absorption peaks of functional groups.The diffuse reflectance spectra were scanned over a range of 400–4000 cm-1.Elemental analysis was performed on the Thermo Scientific FLASH 2000 CHNS/O Elemental Analyzer.Thermogravimetric analyzer(TGA,Perkin-Elmer Instruments Pyris,USA) was used to investigate the thermal stability of the microspheres.Under nitrogen atmosphere,the temperature rose from room temperature to 850°C with a heating rate of 10oC·min-1.Nitrogen adsorption tests were conducted using a specific surface area and pore-size analyzer (ASAP 2020 M,Micromeritics,Norcross,GA,USA).
3.Results and Discussion
In solvothermal precipitation co-polymerization,a mixture of functional monomer,crosslinker and initiator was added into a close vessel and the temperature was then increased up to 85 °C that was higher than the boiling point of the solvent.Based on our previous study [45],with the polymerization temperature increased from 85 °C to 200 °C,the polymer particles tended to more regular spherical shape,but the more aggregated particles were observed floating in the solvent,together with a dramatic decrease in the microsphere yield.Another factor that significantly affected the monodispersity of the forming microspheres is initiator type and its concentration.In the range of 1%to 8%(mass),the greater initiator AIBN concentration led to the more regular PDVB microspheres.In this study,we fixed polymerization temperature at 85 °C,and chose AIBN as initiator and AIBN concentration at 8% as well in the following experiments.
3.1.Monodisperse microspheres containing epoxy group
Table 1 and Fig.1 display the effect of the monomer loading amount ranging from 5 % to 50% on the particle morphologies,where the molar ratio of GMA to DVB was fixed at 20/80.When the monomer loading is increased from 5%to 20%,the narrow dispersed microspheres are obtained (Fig.1a–c).The microsphere yield increases from 67.7% to 94.7%,and the microsphere size increases from 4.184 μm to 5.811 μm in the same range of monomer loading.On further increasing the monomer loading up to 30%,the production falls into two parts.The bottom part consists of uniform-sized microspheres with a PDI of 1.030 and 72.8% yield(Fig.1d),while the upper part presents aggregation of polydisperse particles with 16.7%yield(Fig.1d insert).When the monomer concentration is increased up to 50%,a space-filling macrogel composed of small-sized particles is observed (Fig.1e).It should be noted that our method gives rise to larger microsphere size,comparable uniformity and higher microsphere yield in the fabrication of poly(GMA-DVB) microspheres compared with other precipitation polymerization methods(Table S1).Specially,the microsphere size of the obtained poly(GMA-DVB)particles reaches in the range of 5–10 μm,which fulfills the typical requirement of stationary for high performance liquid chromatography.
These phenomena are similar to that observed in synthesis of PDVB particles by SPP [45].The obtained microspheres were improved on the uniformity with the monomer concentrations increased from 5 % to 30 %,which is distinctively different from the results obtained by traditional precipitation polymerization[5,7,9,13].Two factors should be mainly responsible for this significantly enhanced monomer concentration: one is the closed apparatus with high temperature and high pressure; the other is the quiescent polymerization process without any stirring or shaking.According to the pioneer research by St?ver group [47],particle growth is based on a ‘‘swollen layer” mechanism in precipitation polymerization of DVB.In the early stage of polymerization,oligomers grow up to the critical length or they aggregate,resulting in precipitation out from the mixture to form stable nuclei that have core–shell like structures: a highly crosslinking core and a lightly crosslinking shell.Due to solvency power,the lightly crosslinking shell can swell by monomer,oligomer,and solvent; residual pendant double bonds in this swollen layer can capture monomers and oligomers to form a larger particle.At a relative high monomer concentration(below 20%),a high temperature increases the solubility of both monomers and oligomers in solvent and thus retards the nucleation,and the quiescent way could prevent monomers and oligomers from rapidly colliding and subsequently aggregating; At a higher monomer concentration (above 20%),stronger interactions among nuclei disrupt the auto-steric self stabilization of the nuclei,leading to severely aggregation.A similar trend is observed in the series of particles prepared by different molar ratios of GMA to DVB (Table 1 and Fig.1f–h).With addition of GMA,the surface of the obtained particles became stickier as the amount of the pendent double bonds decreased,as a result of stronger interactions among monomers,oligomers,and particles.Self-steric stabilization was therefore lacking in supporting independence of particles.
3.2.Core-shell like structures of epoxy-contained microspheres
Core-shell like structures of epoxy-contained microspheres are confirmed by energy-dispersive spectrometry (EDS) as shown in Table 2 and Fig.S1.EDS tests based on SEM can reflect element type and distribution within 10 nm depth surface of the sample.The GMA contents on the surface of particles are higher than those of the feed,indicating that the functional monomer is richer in the shell part.Such core–shell structure is further confirmed by swelling tests and TGA tests(Fig.2).After being treated by THF,the poly(GMA-DVB) microspheres shrank slightly to the smaller sizes(Fig.2a–f),suggesting that the loose structures located in the surface of the microspheres [33].Hierarchical thermal gravimetric curves could be relative to the heterogeneous structured copolymers that had highly crosslinked domain and lightly crosslinked one(Fig.2g).To further observe the core shell structure,TEM tests were performed.Both a dark core and a softer shell can be seen on the poly(GMA-DVB) microspheres as shown in Fig.2h,probably indicating DVB-rich part located in the core and GMA-rich part located in the shell,respectively (Fig.2h).
3.3.Microspheres containing other functional groups
To investigate the versatility of solvothermal copolymerization,three functional monomers (LMA,MAA,andHEMA) varying with lower or higher solubility parameter than GMA were chosen to further discuss the effect of the functional monomer on the particle formation.The results in Table 3 and Fig.3 revealed that different functional monomers largely affected the morphologies of the particles.Solubility parameters of the used monomers,crosslinker and solvents are listed in Table 4.In com-parison with PDVB microspheres prepared by SPP in our previous study [45],addition of low polar monomer,LMA (δ=17.22),did not affect the monodispersity of the polymer microspheres in the range of 20%–50% LMA mole fraction; addition of middle-polar monomer MAA (δ=20.53) led to a bimodal microsphere size distribution,but the microsphere monodispersity retrieved when high MAA fraction in the range of 40%–60%was involved;addition of another middle-polar monomer,GMA (δ=21.41),also resulted in narrow-dispersed microspheres at lower GMA fraction,while the monodispersity of the microspheres lost at higher GMA fraction;addition of polar monomer,HEMA(δ=23.88),brought about a broad size distribution,and at higher HEMA fraction no regular particles were found due to the strong solvency as a result of similar solvent parameter between HEMA and acetonitrile (δ=24.4).Another distinctive change lay in that addition of low polar monomer gave rise to smaller-sized microspheres (around 3 μm),but high polar monomer led to larger-sized microspheres (over 5 μm),which was also possibly attributed to the interaction of the polymers and the solvent.With an increase in the polarity of the monomer,the difference in solubility parameter between the monomer mixture and the solvent will be decreased,leading to the extension of the nucleation that brings about a smaller amount of stable nuclei.The particle sizes turn out to be larger in accordance with this trend.Meanwhile,the formed nuclei become stickier under such stronger interactions,and thus are easier to aggregate,resulting in broader size distribution or coagulation.

Table 1Polymerizations of GMA with DVB in acetonitrile
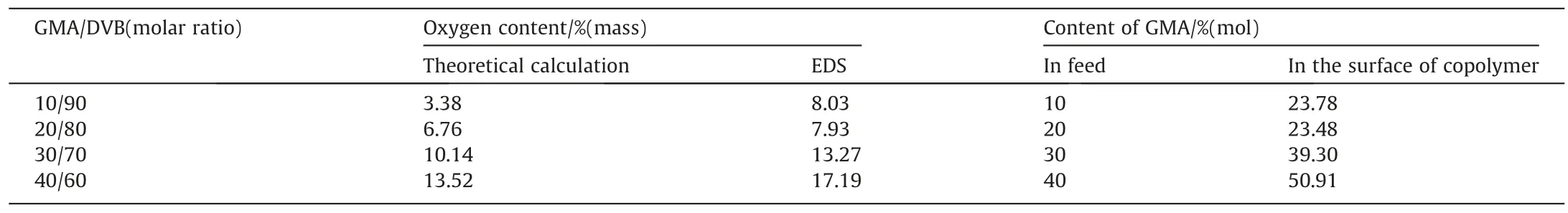
Table 2Compositions of poly(GMA-DVB) particles determined by EDS
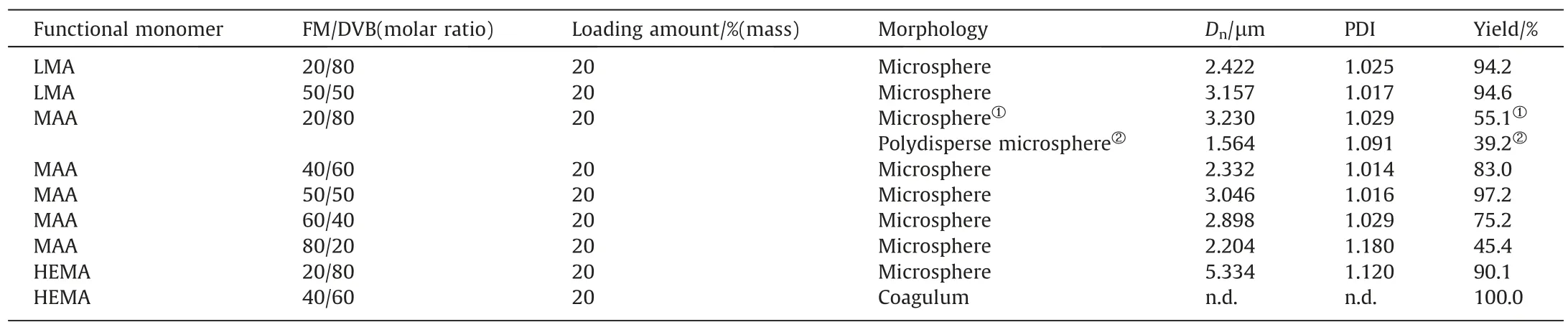
Table 3Polymerizations of functional monomers with DVB in Acetonitrile
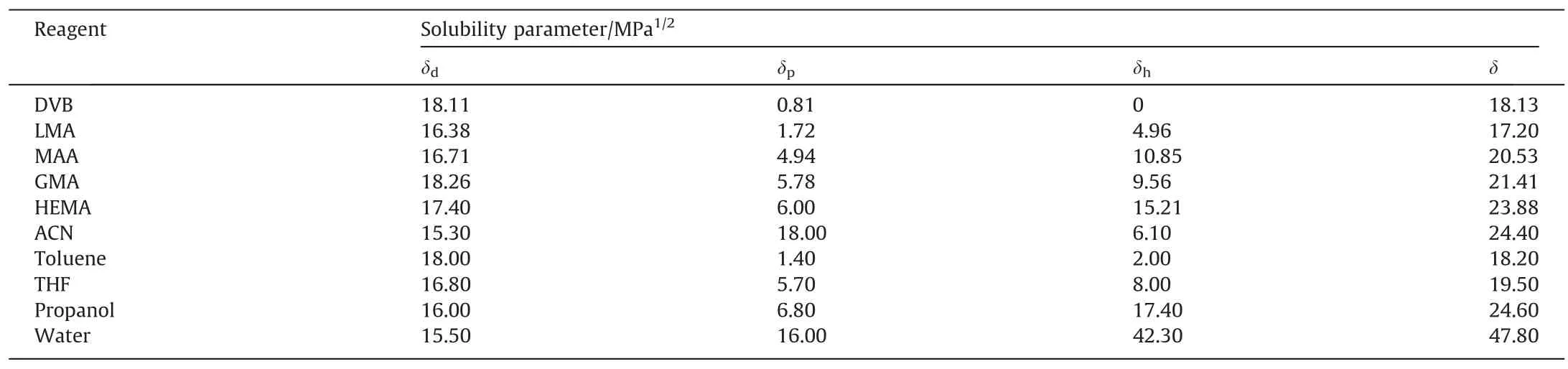
Table 4Estimated Hansen three-dimensional solubility parameters of monomers and solvents at 25 °C
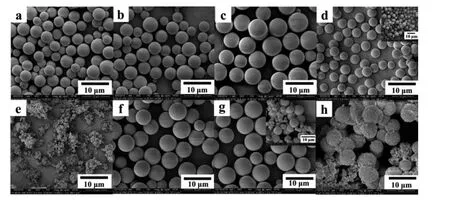
Fig.1.SEM images of the poly(GMA-DVB)particles prepared in acetonitrile at different monomer loadings:(a)GMA/DVB,20/80,5%,(b)GMA/DVB,20/80,10%,(c)GMA/DVB,20/80,20%,(d)GMA/DVB,20/80,30%,inset of(d)shows the upper part,(e)GMA/DVB,20/80,50%,(f)GMA/DVB,10/90,20%,(g)GMA/DVB,30/70,20%,inset of(g)shows the upper part,(h) GMA/DVB,40/60,20 %.
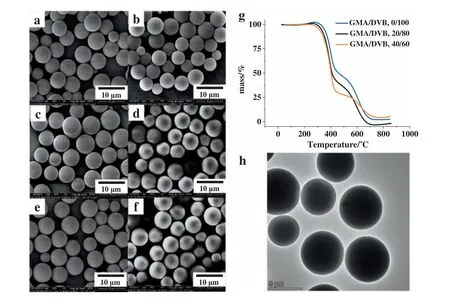
Fig.2.SEM images of poly(GMA-DVB)microspheres before and after swelling in THF:(a,b)GMA/DVB,10/90,(c,d)GMA/DVB,20/80,(e,f)GMA/DVB,30/70.(g)TGA curves of poly(GMA-DVB) microspheres with varied molar ratios of GMA to DVB.(h) TEM image of poly(GMA-DVB) microspheres,GMA/DVB,30/70.
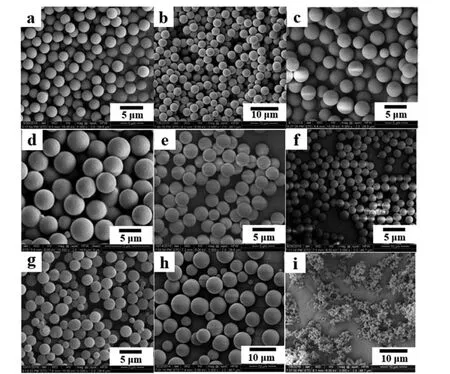
Fig.3.SEM images of the particles prepared with varied molar ratios of functional monomer to DVB in acetonitrile,(a)LMA/DVB,80/20,(b)LMA/DVB,50/50,(c)MAA/DVB,20/80,(d) MAA/DVB,40/60,(e) MAA/DVB,50/50,(f) MAA/DVB,60/40,(g) MAA/DVB,80/20,(h) HEMA/DVB,20/80,(i) HEMA/DVB,40/60.
3.4.Cosolvent tuning microsphere morphology
A key control in precipitation polymerization is the choice of solvent or solvent composition [1,2,4].A proper solvent can dissolve monomer but precipitate out the corresponding polymer to form monodisperse microspheres.For an assigned system including specific monomer and crosslinker,screening the solvent is a time-consuming process and is lack of a instructive protocol up to date.In synthesis of PDVB microspheres,acetonitrile was demonstrated to be an appropriate solvent that met solvency condition for maintaining the monodispersity of the particles [4].Based on previous studies by St?ver group [5]and Igrum group[9],we designed to add a small amount of cosolvent into acetonitrile to alter solvent parameters.Four solvents miscible with acetonitrile,i.e.,toluene,THF,propanol and water were chosen as the cosolvent to study the effect on the morphologies of the particles.
All of the resultant particles copolymerized by LMA and DVB settle down at the bottom of the vessel,and they are narrowdispersed microspheres except in the mixture of acetonitrile and water used as the solvent,where 68.6% aggregated particles float on the top layer of the solution as seen from Table 5 and Fig.4ad.As for copolymerization of MAA with DVB,all of the resultant particles separate into two parts,i.e.,narrow-dispersed microspheres with diameters of 3.4–4.6 μm locating at the bottom of the vessel,and much smaller microspheres with diameters of around 1 μm suspend in the remaining solvent (Table 5 and Fig.S2).It should be noted that the amount of the smaller poly(MAA-DVB) microspheres decreased from 33.0% to 9.6% with an increase in the polarity of the cosolvent.As for copolymerization of GMA with DVB,all of the resultant particles separate into two parts,i.e.,narrow-dispersed microspheres locating at the bottom of the vessel,and aggregated particles floating in the top layer(Table 5 and Fig.S2).The bottom microspheres display a decreasing PDI with an increase in the polarity of the cosolvent.This trend was more significant in the case of copolymerization of HEMA with DVB.With 10% toluene added,the production is a monolithic gel;with 10% THF added,77.3% poly(HEMA-DVB) particles are of spherical shape with rough surface,and 19.0%particles are coagula floating in the top layer;with 10%propanol added,56.8%particles are beaded particles and their surfaces become much smoother though 39.7% particles are found in coagulation; with 2% water added,only one type of particles,i.e.,microspheres,is obtained with PDI of 1.049 and 94.3% particle yield as well (Table 5 and Fig.4e and f).With higher than 2%water added,all types of copolymers of mathacrylic monomers with DVB present severe coagulum(data not shown).
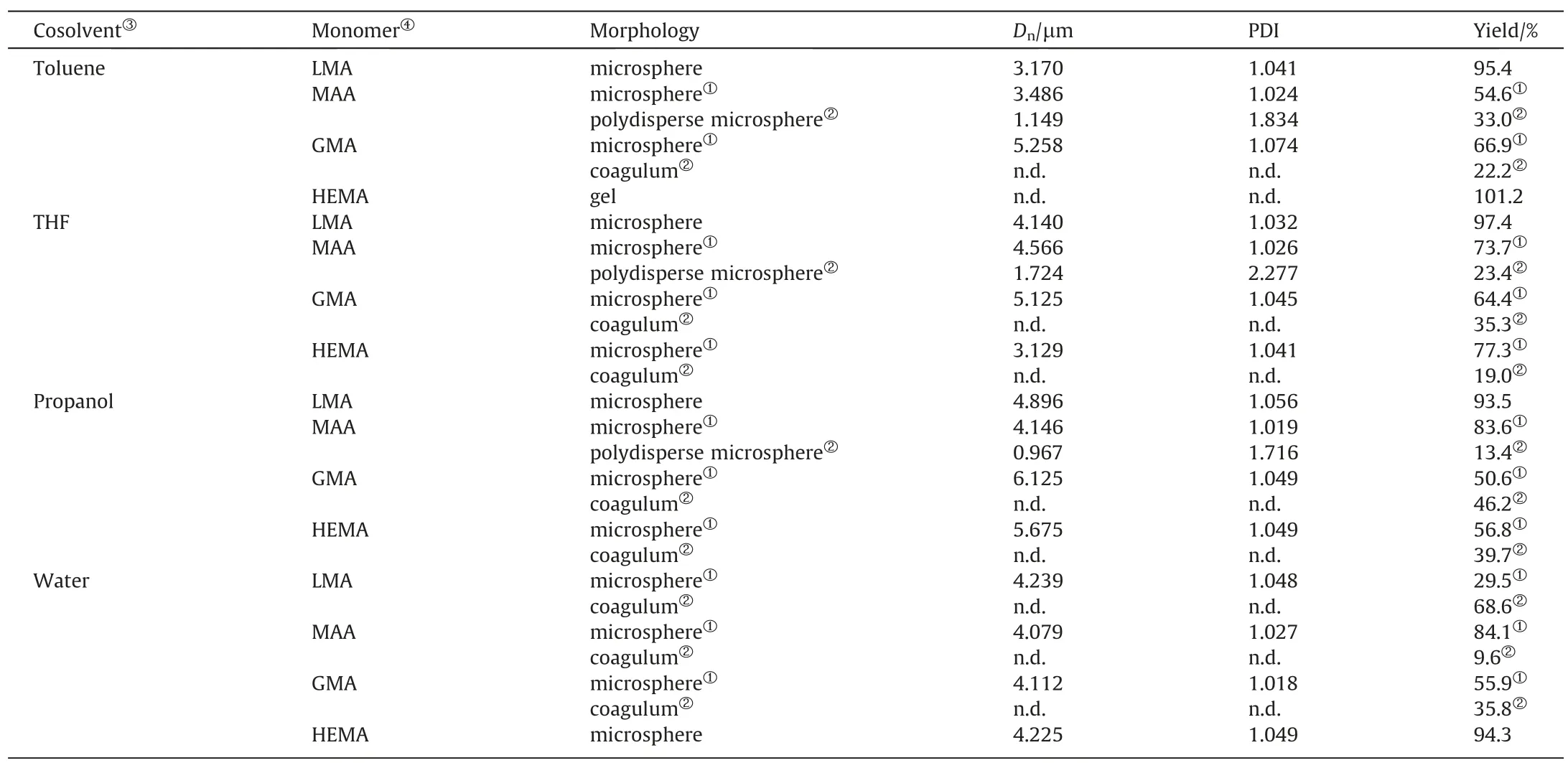
Table 5Polymerizations of functional monomers with divinylbenzene in different admixture of acetonitrile and cosolvent
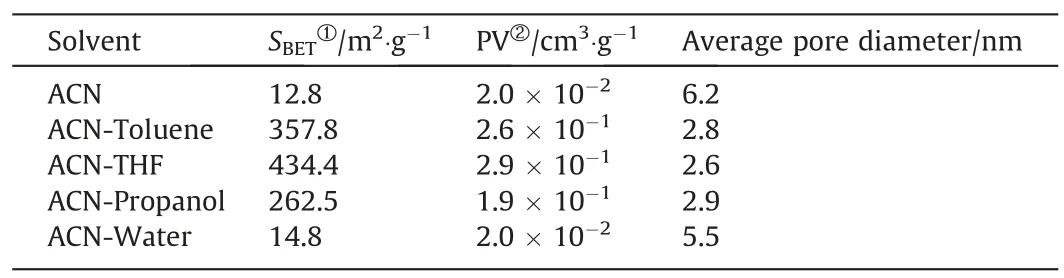
Table 6Pore characteristics of poly(GMA-DVB) particles prepared in different solvents
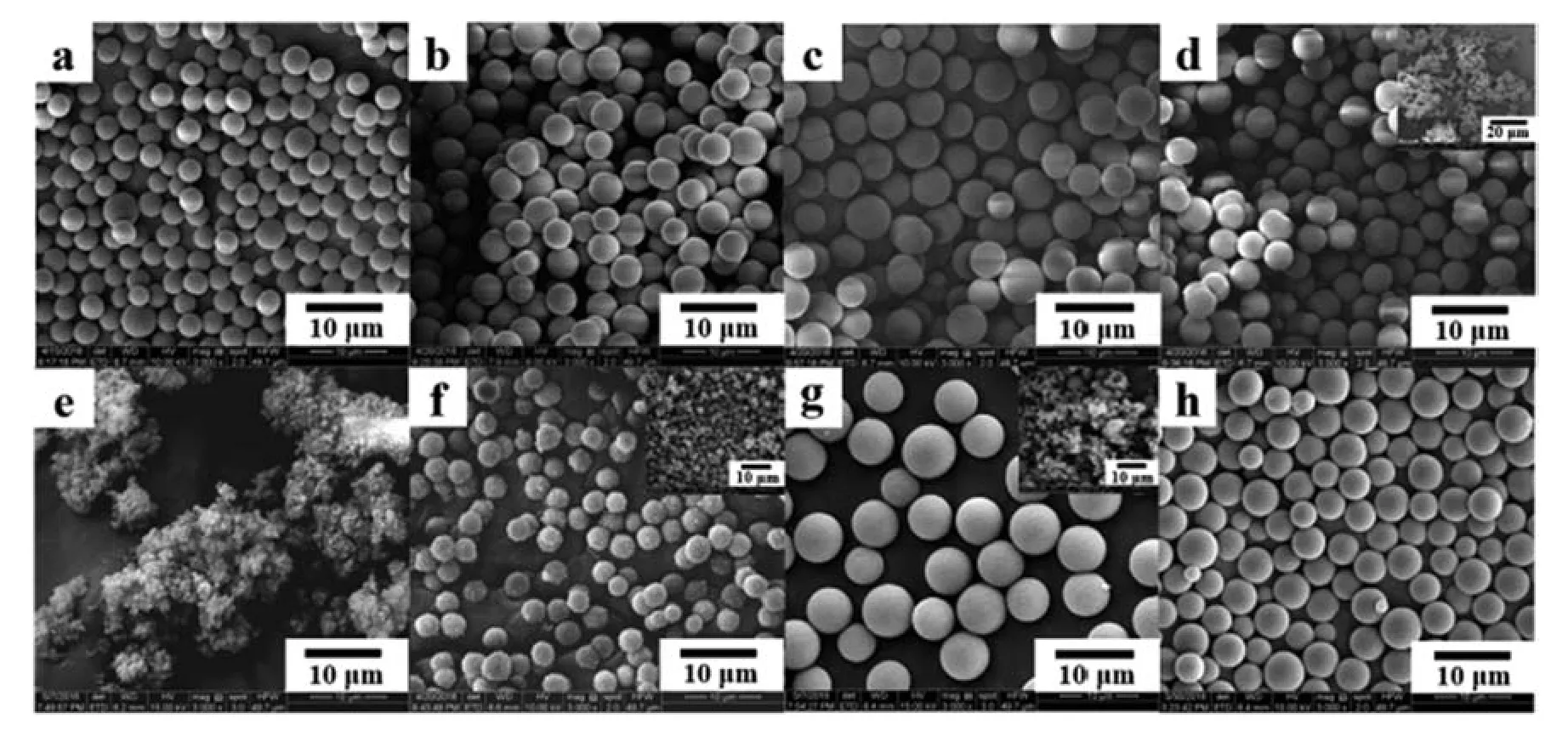
Fig.4.SEM images of poly(LMA-DVB)(a–d)and poly(HEMA-DVB)(e–h)particles prepared in acetonitrile with different cosolvents:(a,e)toluene,(b,f)THF,(c,g)propanol,(d,h) water.The volume ratio of cosolvent to acetonitrile was 10%,except the mixture of water-acetonitrile in which the volume ratio was 2%.
These results strongly suggest that addition of co-solvent is a useful way to control the morphologies of the particles in solvothermal precipitation co-polymerization.A nonpolar solvent is possible positive to a nonpolar monomer to form copolymerized microspheres,and,similarly,a polar solvent is possible positive to a polar monomer to form copolymerized microspheres.A rule of thumb in microsphere preparation by precipitation polymerization is deemed that the Hildebrand solubility parameter of the solvent or the solvent mixture should be 3–5(MPa1/2)away from that of a polymer[49].The difference in solubility parameters can be readily adjusted by addition of co-solvent to fulfill the well-accepted rule.However,under the same recipes in our cases,there were two types of obtained particles,i.e.,floating upper part and lying bottom part,in the binary solvent mixture.In order to further investigate the formation of the particles,we compared the difference of the two types of particles in FT-IR absorption.The intensity ratios of FT-IR absorption peak intensity at 1730 cm-1corresponding to ester group (Iester) to that at 710 cm-1corresponding to phenyl group(Iphenyl)on the copolymers are shown in Fig.5.The intensity ratios of the upper copolymers are greater than those of thecorresponding bottom particles in all recipes,indicating that the functional monomers are richer in the upper part compared to the bottom part.These results are further confirmed that the pendant double bonds can play very important role in the stabilization of the formed particles.
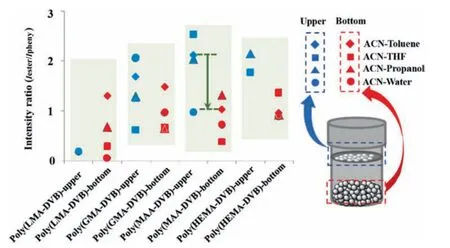
Fig.5.The intensity ratios of the FT-IR absorption peak intensity at 1730 cm-1 corresponding to ester group(Iester)to that at 710 cm-1 corresponding to phenyl group(Iphenyl)on the copolymers prepared in acetonitrile with different cosolvents.The volume ratio of cosolvent to acetonitrile was 10%,except the mixture of water-acetonitrile in which the volume ratio was 2%.
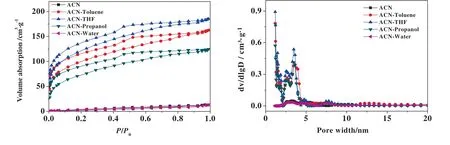
Fig.6.Pore size distribution of poly(GMA-DVB)particles prepared in different solvents measured by nitrogen adsorption(closed)/desorption(open)isotherms of samples at 77.3 K,and pore size distribution curves calculated using DFT method.The volume ratio of cosolvent to acetonitrile was 10%,except the mixture of water-acetonitrile in which the volume ratio was 2%.
Co-solvent possibly gives rise to more porosity of microspheres in precipitation polymerization.When acetonitrile alone was used as solvent,non-porous poly(GMA-DVB) particles were obtained(Fig.6 and Table 6),similar to PDVB particles in the previous study[45].With a small amount of water added,non-porous particles were also obtained.When a low polar solvent was introduced,high surface area of porous poly(GMA-DVB) particles were produced.Especially,when THF was used as co-solvent,the highest surface area achieved 434.4 m2·g-1.The porous formation is probably due to the increased compatibility between the polymers and the binary solvents.When a poor solvent,such as water,was used as cosolvent,the phase separation occurred at the early stage of the reaction.The oligomers aggregated and quickly precipitated out the reaction medium.As a result of a stronger repulsive force,the solvent molecules were squeezed out the polymer network during polymerization.The non-porous structures were observed in the final polymers.By contrast,when a fixed amount of a good solvent,such as THF and toluene,replaced some acentonitrile,the nucleation would be postponed and the solvent molecules were easily trapped in the polymer networks due to a better compatibility.After desolution treatment,the porous structures were left in the polymer particles.These observations are in agreement well with that in the previous study by St?ver group [50].
4.Conclusions
A series of micron-sized highly crosslinked polymeric microspheres containing epoxy,lauryl,carboxyl and hydroxyl groups were successfully obtained at 20% monomer loading by solvothermal precipitation co-polymerization.The effects of monomer loading,monomer composition and solvent composition on the morphologies of the formed particles were studied.Significantly different from the observations in traditional precipitation polymerization,four types of functional microspheres could maintain their uniformity when the monomer loadings were increased up to 20%and the microsphere yields could achieve above 90%simultaneously.Obtained from EDS,TGA,TEM and swelling tests,the results revealed that the formed poly(GMA-DVB) microspheres could be of core–shell like structures,of which DVB was rich in the core and GMA was rich in the shell.Addition of a small amount of co-solvent into acetonitrile can effectively improve the uniformity of the obtained particles,especially for poly(HEMA-DVB).Porous microspheres with surface area higher than 400 m2·g-1were obtained when THF was used as the co-solvent.The resultant microspheres are of tunable functionality,narrow size distribution,several microns in diameter and controlled porous structures,which are highly expected in use of as stationary for liquid chromatography or solid phase extraction.These results suggest that solvothermal precipitation co-polymerization is a potential method for large scale-up preparation of various monodisperse microspheres with pure surface.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (51873079) for financial support.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.09.036.
 Chinese Journal of Chemical Engineering2021年6期
Chinese Journal of Chemical Engineering2021年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes
- Synthesized graphene oxide and fumed aerosil 380 dispersion stability and characterization with partially hydrolyzed polyacrylamide
- Synthesis and characterization of caprolactone based polyurethane with degradable and antifouling performance
- Removal of lead (Pb(II)) and zinc (Zn(II)) from aqueous solution using coal fly ash (CFA) as a dual-sites adsorbent
- Catalytic performance improvement of volatile organic compounds oxidation over MnOx and GdMnO3 composite oxides from spent lithium-ion batteries:Effect of acid treatment
- Application of fracturing technology to increase gas production in low-permeability hydrate reservoir:A numerical study
