Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes
Zhenghui Liu,Jun Xiang,Xiaoli Hu,Penggao Cheng,Lei Zhang,Wei Du,Songbo Wang,Na Tang
Tianjin Key Laboratory of Brine Chemical Engineering and Resource Eco-utilization,College of Chemical Engineering and Materials Science,Tianjin University of Science and Technology,Tianjin 300457,China
Keywords: PPSU ultrafiltration membrane Ternary-phase diagram Coagulation bath conditions Sponge-like structures
ABSTRACT Polyphenylsulfone (PPSU) ultrafiltration membrane with different structures was prepared by nonsolvent-induced phase separation.The effects of coagulation bath conditions(concentration and temperature)on membrane morphology,pure water flux,pore size,porosity,and contact angle were studied and discussed based on ternary-phase diagrams.Results indicated that water had stronger coagulant power than ethanol,and that the morphology of the membrane prepared from the polyphenylsulfone/1-methyl-2-pyrrolidinone/H2O (PPSU/NMP/H2O) system had finger-like structures.Conversely,sponge-like structures were observed for the PPSU/NMP/(NMP-H2O) and PPSU/NMP/(70NMP-EtOH-H2O) systems.Ethanol also greatly influenced on membrane structures.According to the Scanning electronic microscopy (SEM) image,the composition (mass fraction) of casting solution is 16% PPSU-84% NMP and the coagulation bath consisting of 70%NMP-26%H2O-4%C2H5OH.Meanwhile,the PPSU ultrafiltration membrane with spong-like was prepared under 8 ℃coagulation bath.The formation of sponge-like structure reduces the pure water flux of ppsu membrane from 488.39 L·m-2·h-1 to 36.04 L·m-2·h-1.It also reduces the gas permeability,porosity,and pore size of the membrane.The addition of ethanol and NMP into the coagulation bath increases the roughness of the PPSU ultrafiltration membrane and reduces the hydrophilicity of the membrane.
1.Introduction
Non-solvent induced phase separation (NIPS) method is extensively used to produce asymmetric porous membranes due to its excellent ability to control membrane structures and properties[1–6].In the precipitation process of liquid membranes,the amount of non-solvent in the casting solution increases due to the two-way exchange between solvent and non-solvent inducing the system become unstable,Consequently,liquid–liquid demixing occurs and different membrane structures form.The exchange rates between non-solvent and solvent in casting solution govern the ultimate membrane structure[7–9].The composition and temperature of the coagulation bath (CB) greatly affect the structure and performance of the membrane [9,10].Xuet al.[11]reported that increased CB temperature results in greater formation of macrovoids and more porous structures.Deshmukhet al.[12]studied that increased ethanol content in the CB slowly changes the membrane pore structure from finger like to sponge-like.Increased viscosity of casting solution by the addition of additives(e.g.,PEG and PVP) to the casting solution can also reduce the exchange of solvent in the casting solution with non-solvent from the CB[13–15].Thus,we can select the best balance point between thermodynamics and kinetics to control the membrane structure.Pliskoet al.[16]reported a novel method for the preparation of high-flux PPSU ultrafiltration membranes.According to the ternary-phase diagrams of the PPSU–PEG–NMP systems obtained,when the composition (mass fraction) of the casting solution is 20% PPSU–15% PEG6000–65% NMP and the CB temperature is 70°C,the flux is close to 500 L·m-2·h-1.Yinet al.[8]used PPSU flat sheet membranes with NIPS at 50 °C.They found that with increased PVP Mw,the membrane structure shifts from fingerlike to sponge-like.At a higher concentration (10%,mass),with PVP Mw greater than 360 kDa,PPSU membrane does not exhibit any finger-like structure and instead shows sponge-like structure.For membranes with finger-like structures,the pure water flux is high but the mechanical strength is low.Membranes with sponge-like structures are usually used in reverse osmosis,nanofiltration and gas separation.In these processes,membranes with poor mechanical stability are compacted under high pressure,leading to the irreversible loss of flux.Accordingly,in practical applications,sponge-like membranes are more popular due to their high mechanical strength and integrity.
For membranes prepared by phase inversion,the structure is controlled by the thermodynamic and kinetic aspects of three components (polymer,solvent,and non-solvent) [3,17–19].Ternaryphase diagram can enable the study of thermodynamic influence and interaction parameters among components.From the position of a binodal line,the appropriate solvent and non-solvent for the polymer can be selected to adjust membrane structures.The binodal line divides a ternary-phase diagram into a stable region and an unstable one,as shown in Fig.1 [20].During immersion precipitation,liquid–liquid demixing can be split into spinodal decomposition and nucleation and growth separation based on passing the critical poin [21,22].If the composition of system exceeds the critical point (path (a)),polymer-lean liquid–liquid separation occurs and a cellular structure forms; conversely,less complete aggregation is achieved in polymer-rich liquid–liquid separation (path (b)).From the critical point to unstable region(path (c)),the formation of a bicontinuous structur is facilitated.For crystalline polymers,liquid–liquid separation and solid–liquid separation in phase inversion occur,and the membrane structure is determined by which one is dominant.At present,the ternaryphase diagram of components is still a key point in analyzing the formation of membrane structure.
The ideal membrane material should have high flux,excellent anti-fouling performance,and sufficient mechanical strength.Polyphenylsulfone (PPSU) is a promising sulfone polymer extensively used in membrane material ultrafiltration and nanofiltration.Its chemical structure is show in Fig.2.Due to the conjugated structure connected by two benzene rings,the rigidity of a PPSU material can be maintained[24].Moreover,PPSU’s glasstransition temperature of 220°C is higher than that of polysulfone(PSF) at 190 °C and almost equal to that of polyethersulfone (PES)at 225 °C [16,25].PPSU also has higher organic solvent resistance than PSF and PES[16],like ethers,ketones,aromatic amines,acids,alkali,nonionic surfactants and aromatic hydrocarbons,This feature can be applied in the mentioned solution environment,and even in support membranes of nanofiltraion membranes and reverse osmosis membranes.
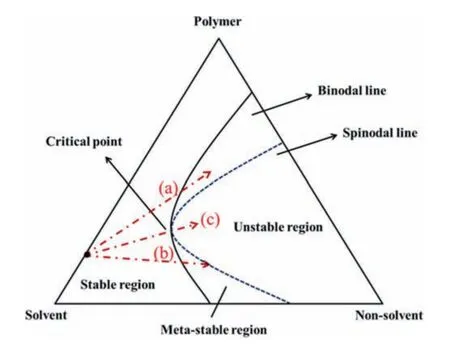
Fig.1.Mechanism of membrane formation [23].
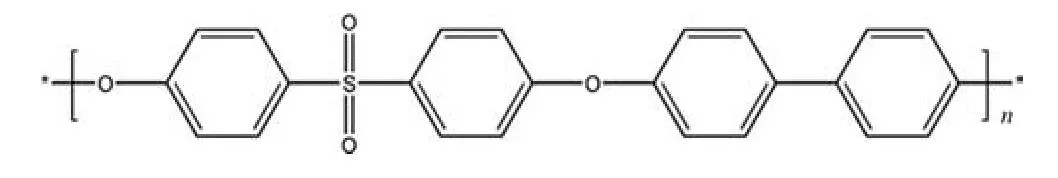
Fig.2.Chemical structure of Polyphenylsulfone (PPSU).
PPSU membrane with sponge-like structures can reportedly be obtained from high-temperature casting solutions.In the present study,PPSU ultrafiltration membranes with sponge-like structure were prepared by NIPS technique below room temperature.The relationship between the morphology and performance of PPSU membranes and phase diagrams was analyzed.To the best our knowledge,phase diagrams of the PPSU/NMP/non-solvent systems have not yet been reported.Herein,we drew the phase diagram of the ternary system through the cloud-point titration method combined with Flurry Huggins theory.According to the ternary-phase diagrams,we optimized the membrane-fabrication process by adjusting the CB composition and temperature for fabricating PPSU membranes with sponge-like structures.
2.Experimental
2.1.Materials
Polyphenysulfone (PPSU,Mw=51,000 g·mol-1) was purchased from BASF,Inc.(Germany).N-methyl-2-pyrrolidone (NMP) as solvent and ethanol(EtOH,purity of 99.7%)as non-solvent were supplied from Tianjin Guangfu Chemical Reagent Co.Ltd.(Tianjin ,China).Non-woven fabrics was used as a support layer supplied from Hokuetsu Kishu Paper Co.Ltd.and its parameters show as Table 1.All DI water were supplied by home-made (resistivity: 6 MΩ·cm).
2.2.Phase diagrams
The thermodynamic equilibrium of a memet al.brane-formation process is explained by a phase diagram.The phase diagram of the PPSU/NMP/non-solvent was obtained by simple titration method[26].First,different mass fraction of PPSU(1%,2%,3%,4%,5%)were dissolved in the solvent NMP for 6 h until a homogeneous solution formed.Then,titrations with known compositions were added dropwise to the mechanically stirred PPSU solution at the same temperature until incipient turbidity was observed.The cloud point was allocated when the solution turbidity remained for a few minutes.
2.3.Preparation of PPSU solutions
PPSU was dried at 65°C for 24 h in a vacuum oven before used.To prepare the membrane-casting solution,16% PPSU was dissolved in NMP solvent followed by stirring for 12 h at 60°C to form a homogeneous mixture before degassing at room temperature for 12 h.
2.4.Membrane preparation
PPSU membranes were prepared by wet-phase-inversion method.The casting solution was poured onto a non-woven with an initial thickness of 150 μm and instantly immersed in a CB with designated temperature and composition for 5 min.the membranes were then transferred into a container with roomtemperature deionized water to completely remove the residual solvent.The temperatures and compositions of several mixtures of the CB used are listed in Table 2.

Table 1Parameters of Wet-Laid polyester non-woven fabric ‘‘S53”
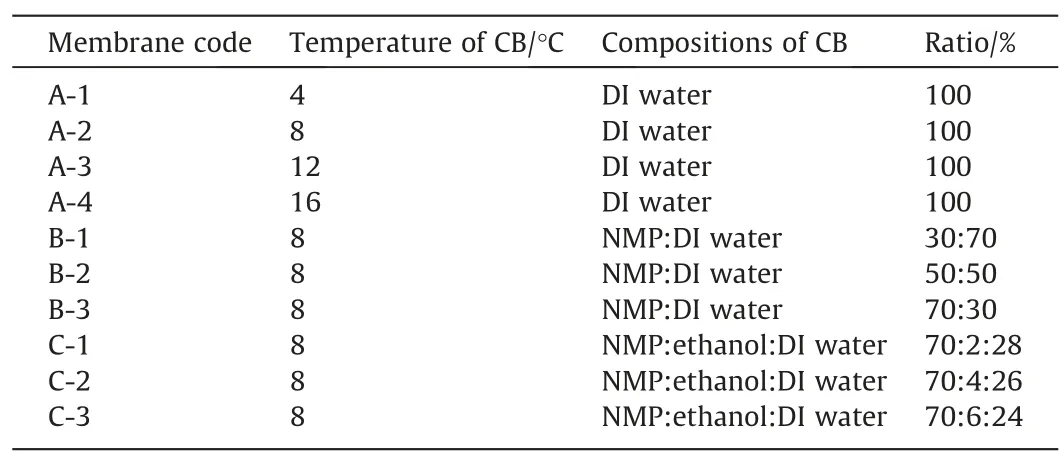
Table 2Formation conditions of the membranes cast from the casting solutions consisting of 16% PPSU-84% NMP

Table 3The effect of CB condition on membranes porosity and mean pore size
2.5.Membrane characterization
2.5.1.Pure water flux
Pure water flux (PWF) of the prepared PPSU flat-sheet membranes was measured using a cross flow apparatus (made in laboratory)with effective membrane area of 28.56 cm2,The velocity of the cross flow is 1.56 cm·s-1as show in Fig.3.The flux measurements were carried out at room temperature,trans-membrane pressure of 1 bar (1 bar=0.1 MPa).Each membrane sample was kept in distilled water for 24 h before experiments.Membranes were compacted for 15 min at 1.5 bar at room temperature before measured.Pure water flux was calculated using Eq.(1):

whereJis the pure water flux (L·m-2·h-1),Vis the total volume of the permeate water (L),Ais the effective membrane area (m2),Tis the permeation time (h).
2.5.2.Scanning electron microscopy (SEM) and atomic force microscopy (AFM)
Morphologies of the PPSU membrane are visualized by SEM(PW-100-019,Phenom PURE,Holland).To obtain the crosssectional images,PPSU membranes were fractured under liquid nitrogen to have a generally consistent and clear break and then dried.The surface and cross section of the membrane were treated with gold spraying in SBC-12 type ion sputtering instrument before SEM analysis.The current was below 10 mA and the vacuum was 6–5 Pa.The surface roughness of ppsu membranes were observed using AFM (Nanoscope IV,American VEECO).Then compare the roughness with the hydrophilicity of the membranes.The size of the test sample is 40 μm×40 μm.The AFM images were analyzed through Nanoscope software.
2.5.3.Contact angle
Water contact angle was measured to indicate the hydrophilicity of membrane surface.It was measured by using a contact angle measuring instrument(DSA100,Krüss,Germany)at room temperature.Membrane samples were cut into appropriate size and fixed onto the glass slide.Using the motor-driven micro syringe,3 μl of deionized water was pumped out from the syring onto the sample surfaces and the direct microscopic measurement of the contact angles was done with the goniometer.For each membrane sample the contact angle was measured at more than 7 different spots,and the reported data of the contact angle represent an average of the measurements.
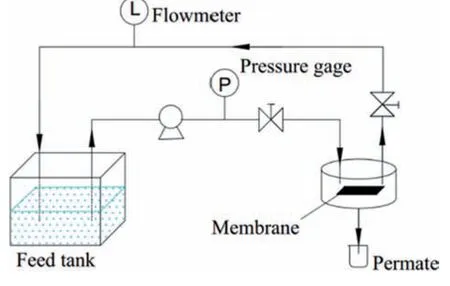
Fig.3.Cross flow set-up diagram of pure water flux.
2.5.4.Porosity
The porosity was determined by soaking the membranes in porefil solution for 24 h.The residual porefil on the surface of the membranes was removed by blotting with tissue paper.Porosity was calculated by gravimetric method as defined in the following Eq.(2):

wheremwandmdis respectively the mass of wet and dry membrane (g),Ais the membrane area (4.91 cm2),lis the membrane thickness (cm),ρ is infiltrating liquid density (1.85 g·cm-3).
2.5.5.Pore size and gas permeability
Pore size and gas permeability of membranes were measured by Porometer PoroluxTM1000(IB-FT GmbH,Germany).Each sample was cut into 13 mm in diameter and wetted by the porefil solution(surface tension of 16.0 dyne·cm-1)as the wetting liquid.Then the fully wetted samples were positioned on the sample holder followed by more wetting on the top of the membrane before the chamber was sealed.Nitrogen gas was then allowed to flow into the chamber steadily.As the pressure of the nitrogen increased,all pores will be emptied from the solution and the membrane were considered dry.Gas pressure and flow rates through the dry sample were also recorded.The pore size distribution was later calculated by the processing software.For gas permeability,membrane was cut into 25 mm in diameter and put into the sample holder.The flux of nitrogen gas was measured under 1 bar.
3.Results and Discussion
3.1.Phase diagrams
The formation of membrane by phase inversion is very close to the phase-separation mechanism of components.Thermodynamic description of porous structure-formation enables the prediction of the conditions(temperature and composition)at which the system separates into two phases [27].In the present study,we drew the phase diagram of the ternary system through the cloud-point titration method combined with Flory-Huggins theory.

Fig.4.The cloud point lines of PPSU/NMP/H2O system at 8°C and 20°C and PPSU/NMP/(70%NMP-30%H2O) system at 8 °C.
The calculated cloud-point dates for the PPSU/NMP/H2O system are shown in Fig.4.the cloud-point curves were close to the PPSUNMP axis,and only a little movement occurred toward the PPSU/H2O axis at 20 °C compared with 8 °C.This result showed that the membranes were easily obtained using PPSU and that the interchange between NMP and H2O was rapid due to the large demixing gap that led to finger-like pore formation [20].Meanwhile,the addition of NMP at non-solvent caused the power of coagulation to decrease and the cloud-point to move away from the PPSU/NMP axis in the PPSU/NMP/(70%NMP-30%H2O) system[18,28].Thus,a transition from instantaneous to delayed demixing occured.
The ternary-phase diagrams of the PPSU/NMP/EtOH system at 8°C are shown in Fig.5.The miscibility gap of the PPSU/NMP/EtOH system was larger than that of the PPSU/NMP/H2O system,which indicated that water had stronger coagulant power than ethanol[20,26].The non-solvent/polymer interaction reveal that ethanol had higher affinity toward PPSU than water based on their solubility parameter (26.5 MPa0.5for ethanol [29],versus 47.8 MPa0.5for water[30]).Analysis of kinematic property showed that the diffusivity of water/NMP (18.0×10-6cm2·s-1) was higher than that of ethanol/NMP(10.5×10-6cm2·s-1),whereas the values of NMP/water and NMP/ethanol were similar [31].Moreover,the cloud-point line of the PPSU/NMP/(70%NMP-EtOH-H2O) system was obtained with a change in the ratio of H2O to EtOH in the presence of 70 %NMP in coagulant at 8 °C.Fig.5 further show that increased ethanol concentration induced the binodal line to move toward the PPSU-non-solvent axis.In other words,the addition of ethanol enlarged the miscibility gap area large and delayed the phase separation.
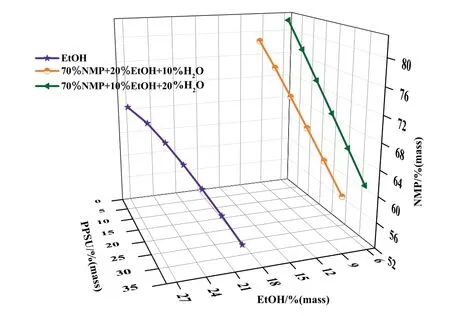
Fig.5.The cloud point lines of PPSU/NMP/EtOH system and PPSU/NMP/(70%NMPEtOH-H2O) system at 8 °C.
3.2.Effect of the coagulation temperature on PPSU membrane morphology
With distilled water as coagulant,the effect of CB temperature on the PPSU membrane structure was investigated for membranes obtained from the 16 % PPSU-84 % NMP system (Fig.6).We found that the membranes had a typical asymmetric structure formed through the NIPS technique by instantaneous demixing mechanism.They comprised a thin dense skin layer,a porous support with finger-like macrovoids,and a small portion with cells.The number of microvoids also gradually increased with increased CB temperature.The CB temperature affected the rate of polymer precipitation and regulated the rate of non-solvent streaming into the polymer solution,thereby determining the structure of porous and their interconnectivity within the membrane structure.Based on the ternary-phase diagram in (Fig.4),the binodal line was close to the PPSU/NMP axis of the PPSU/NMP/H2O system,so only a small amount of water was required to precipitate PPSU and cause instantaneous demixing for forming finger-like structures.Meanwhile,temperature had little influence on the position of the binodal line,and the miscibility of NMP and water with increased temperature,causing the coagulant ability of water to decrease and lead to microvoid formation.This study was according with previous ones [5,32,33],showing that finger-like structures are formed using NNP as solvent and water as non-solvent.
3.3.Effect of the CB compositions on PPSU membrane morphology
According to Fig.6(A-4)and Fig.7,the concentration of NMP in the CB significantly influenced the structure of PPSU membrane with 16 % cross-section at 8 °C.With increased NMP mass radio in CB,the figure-like wall thickened and sponge-like structures gradually replaced figure-like structures from the membrane bottom.When NMP concentration reached 70 %,all figure-like structures transformed into drop-shaped macrovoids.Surface pore density decreased with increased NMP concentration as shown in Fig.8.Demixing is a double-diffusion [34],in which the nonsolvent diffuses into a dope solution and the solvent and additives diffuse into non-solvent[35].Coagulation medium plays an important role in membrane formation through the phase-inversion method.According to the quaternary diagram of PPSU/NMP/(70%NMP- 30%H2O),the binodal approach to the PPSU/H2O axis with NMP addition in coagulant and the demixing gap became narrow.Moreover,increasing the content of solvent in the CB weakened the interaction between coagulant and casting solution.In other words,with increased NMP concentration in the CB,the rate of non-solvent diffusion into the polymer solution slowed down and suppressed the formation of figure-like structures.From the morphologies of PPSU membrane,we found that kinetic factor was more superior than phase equilibrium in the PPSU/NMP/H2O system.Because of the proximity of the position of binodal lines at 20°C and the NMP addition(Fig.4).However,the morphologies significantly differed.Apparently,instantaneous demixing was preferred over slow demixing when NMP concentration was low(<50%),whereas slow demixing dominated when the NMP concentration reached 70 %.
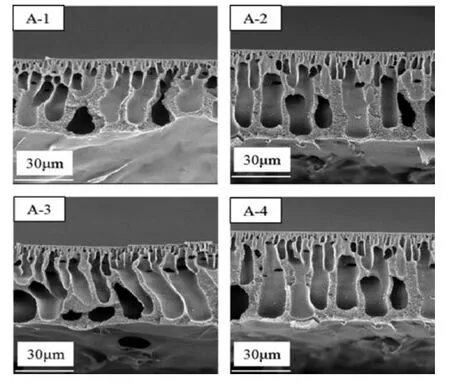
Fig.6.Effect of coagulation temperature on PPSU membrane cross-section.
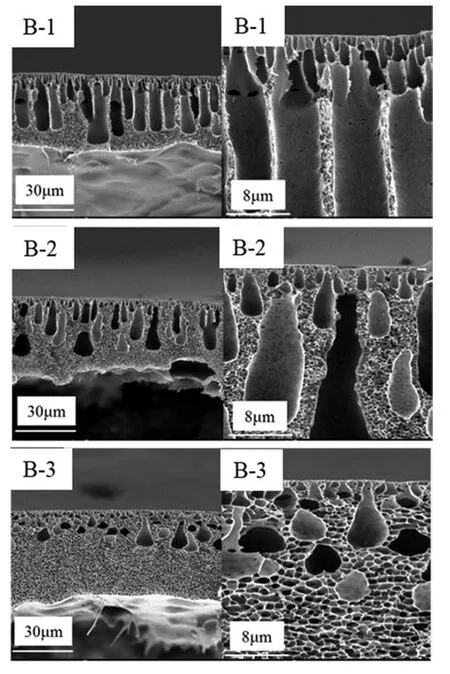
Fig.7.SEM micrographs of the cross-section and enlarged fragments of crosssection of membranes with different the mass ratio of NMP in CB at Tcb=8 °C.
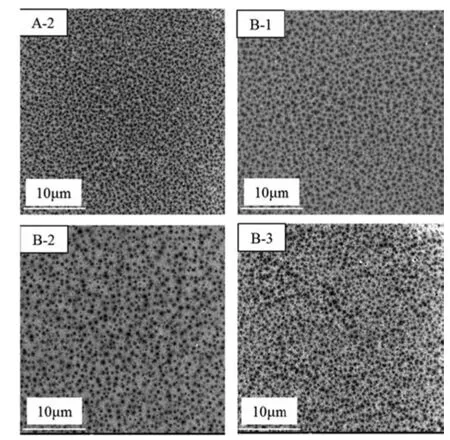
Fig.8.SEM micrographs of the top surface of membranes with different the mass ratio of NMP in CB at Tcb=8 °C.

Fig.9.SEM micrographs of the cross-section and enlarged fragments of crosssection of membranes with different the mass ratio of EtOH in CB at 70%NMP in CB,Tcb=8 °C.
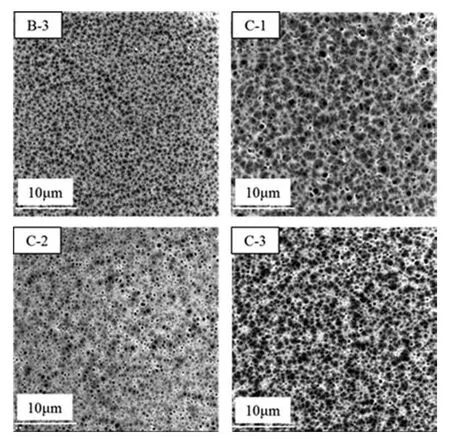
Fig.10.SEM micrographs of the top surface with different the mass ratio of EtOH in CB at 70 % NMP in CB, Tcb=8 °C.
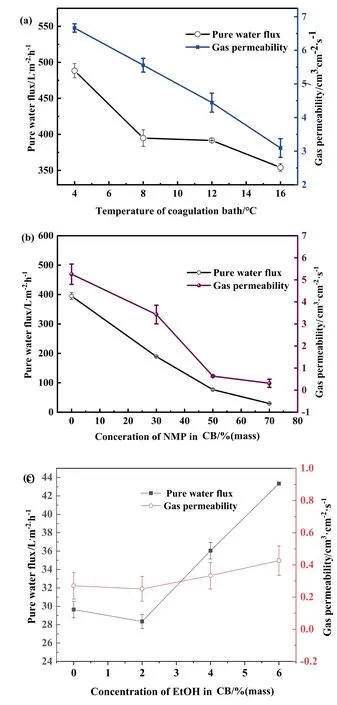
Fig.11.Effect of CB conditions on membrane pure water flux and gas permeability(a)CB temperature,(b)NMP concentration in the CB,(c)EtOH concentration in the CB.
In the presence of 70%NMP in the CB,the effect of ethanol concentration from 0%to 6 %on cross-section and surface of the prepared membranes was investigated.Results are shown in Figs.9 and 10.Fig.9 shows that the ethanol addition resulted in eliminating macrovoids and facilitated the formation of sponge-like pore structures.Meanwhile,the pore size on the membrane surface slightly increased from those shown in Fig.10.This phenomenon was related to the delayed liquid–liquid demixing effect of ethanol and NMP.As mentioned above,the thermodynamics and kinetics of the solvent/non-solvent exchange significantly influenced the type and structure of membrane voids.The binodal line of the PPSU/NMP/EtOH system was close to the PPSU/EtOH axis and the dimixing gap was the smallest from Fig.5,indicating that ethanol can delay phase seperation.At the phase diagram of the PPSU/NMP/(70%NMP-EtOH-H2O) system,the miscibility gap gradually enlarged with increased ethanol amount.Based on phase diagram,increased ethanol amount in the external CB resulted in decreased precipitation rate and induce a change in membrane morphology from finger like to sponge-like [36].Moreover,some hydrogen bonds formed between ethanol and NMP,which reduced the interaction between water and NMP.From Fig.9 further shows that completely sponge-like structure were obtained when the ethanol content was 4 % (mass),indicating that ethanol significantly influenced the PPSU membrane morphology.
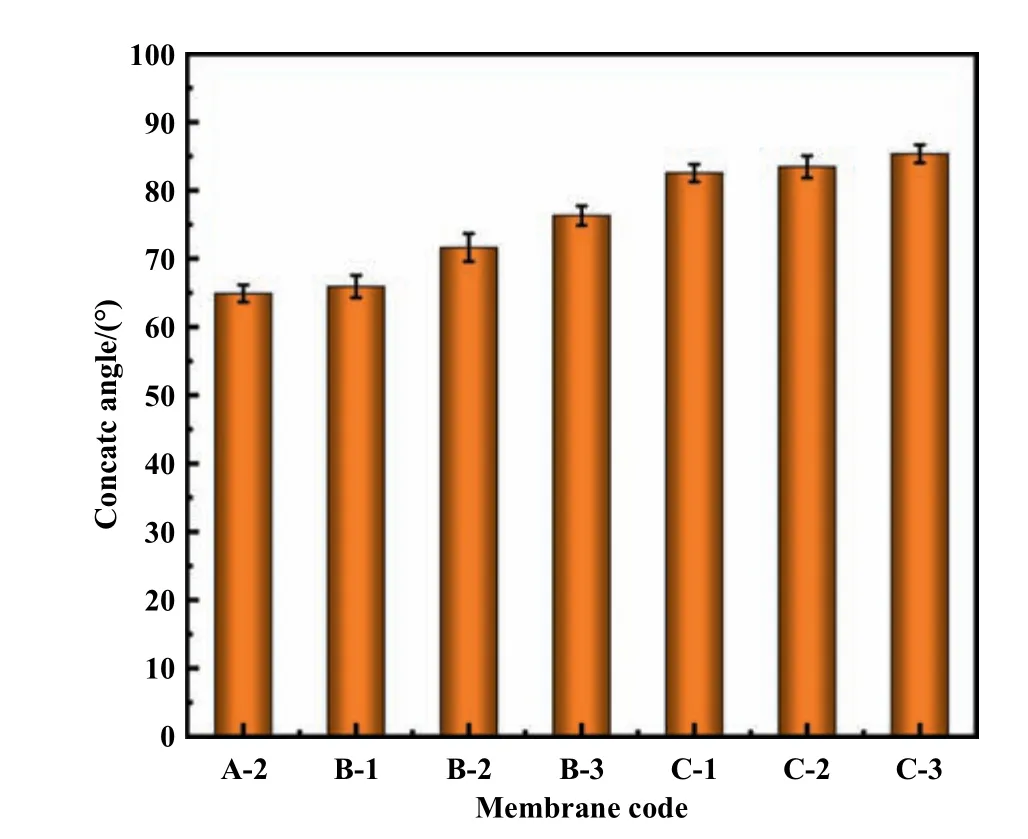
Fig.12.Effect of CB composition on membrane contact angle.
3.4.Permeation studies
The Pure water flux and gas permeability of the prepared membranes are presented in Fig.11.With increased the CB temperature from 4°C to 12°C,pure water decreased from 488.39 L·m-2·h-1to 354.03 L·m-2·h-1,whereas the formation of sponge-like structures caused the flux to decrease to 29.64 L·m-2·h-1.The same phenomenon was observed for gas permeability,when NMP concentration reached to 70 % in the CB,gas permeability was only 0.31 cm3·cm-2·s-1.These measurements confirmed the trend revealed by the SEM images,Increase the temperature of the CB delays the phase separation,which led to the formation of microvoids and reduces the pore size.The formation of sponge-like structures was due to the addition of NMP to the CB,which resulted in lower pure water flux and gas permeability values.Meanwhile,sponge-like structures significantly influenced pore size,as shown in Table 3.A mass of sponge-like structures formation leaded to failure in measuring dry membrane pore size and used the wet membrane.
However,for ethanol,these values increased with increased mass ratio of ethanol in the CB.Flux and gas permeability was 43.34 L·m-2·h-1and 0.43 cm3·cm-2·s-1,respectively,in the presence of 70%NMP and 6%EtOH in the CB.Because the slower precipitation formed a more porous symmetric membrane with a skinless surface.Consequently,pore distribution widened with increased ethanol concentration,and the pore size on the membrane surface increased (Fig.10).The trends of porosity were also in accordance with the membrane morphologies shown in Table 3.
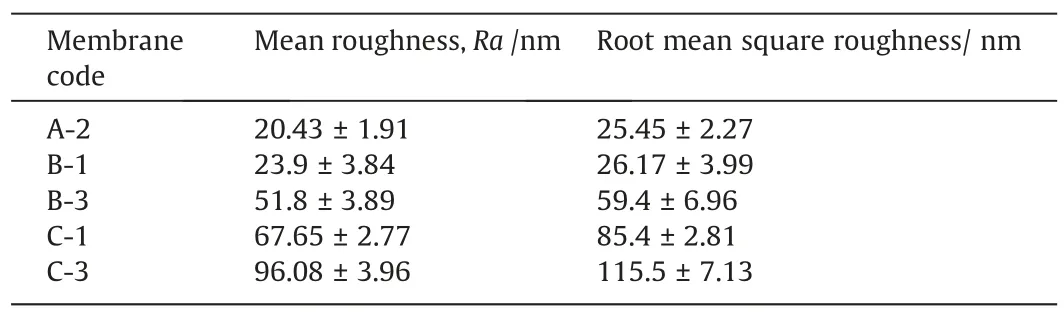
Table 4Roughness parameters in different coagulation bath compositions
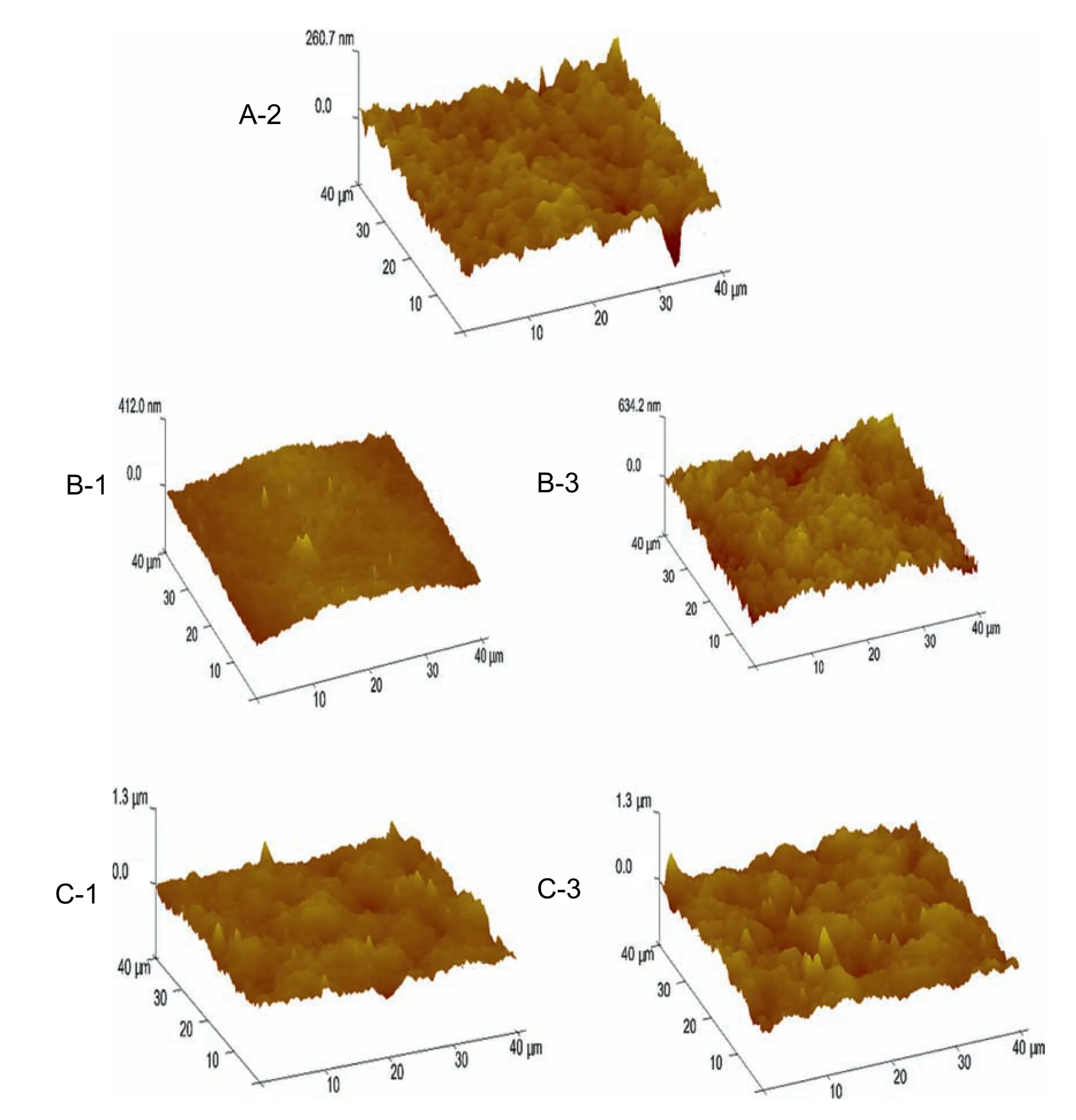
Fig.13.Effect of CB composition on membrane surface roughness.
3.5.Contact angle
The effect of CB conditions on PPSU membrane contact angle is shown in Fig.12.With increased NMP concentration in the CB,the contact angle of membrane increased from 64.88° to 76.30°possibly because the formation of hydrogen bonds among NMP,water and PPSU resulted in the methyl group of NMP being exposed to the membrane surface.Meanwhile,the addition of ethanol decreased the membrane hydrophilicity,and contact angle increased to 85.37° in the presence of 6% ethanol in the CB.Adding ethanol to the CB delayed liquid–liquid demixing,which increased the contact angle possibly duo to the change in membrane surface roughness.Ahmadet al.[37]reported that low-concentration of ethanol in the CB improves membranes hydrophobicity.Fig.13 show the topographic images of the PPSU membranes.The surface roughness values listed in Table 4.With increased NMP mass radio in the CB,the roughness of the PPSU membrane increases from 20.43 nm to 51.8 nm.Some literature reported that the hydrophilicity of membranes are usually influenced by porous surfaces and roughness of membranes [38,39].The increased ethanol content in the CB delayed the phase separation and changed the pore density of the membrane surface,which increased the value of membrane roughness from 67.65 nm to 96.08 nm.Therefore,enhanced membrane surface roughness can reduced membrane surface hydrophilicity by increasing the water contact angle [8].Thus,surface roughness can be increased using ethanol as non-solvent,and the hydrophilicity can be reduced accordingly [8,40].
4.Conclusions
The morphologies and performance of PPSU membranes were investigated based on phase diagrams.Based on the ternary phase diagrams of PPSU/NMP/H2O and PPSU/NMP/EtOH,water was a strong non-solvent and ethanol was a weak non-solvent.Fingerlike structures formed with water as the CB.In the quaternary phase diagram of the PPSU/NMP/(70NMP-30H2O)system,the miscibility gap increased and sponge-like structures gradually replaced finger-like structures from the bottom.The addition of ethanol to the CB delayed the phase separation,resulting in the formation of completely sponge-like structures and inducing the binodal line to move away from the PPSU-NMP axis of the PPSU/NMP/(70NMP-EtOH-H2O) system.
Pore size,pure water flux,gas permeability,and porosity decreased with the increase of CB temperature and NMP concentration in the CB,due to the formation of microvoids and spongelike pores.In the presence of 70 % NMP in the CB,these values only slightly increased with the addition of ethanol.The addition of ethanol to the CB also reduced membrane hydrophilicity of the membrane by increasing the roughness of its surface.We believed that PPSU membrane with sponge-like structures can be used in nanofiltration,reverse osmosis,and catalyst loading processes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by National Natural Science Foundation of Tianjin (18JCZDJC37200),Tianjin University of Science &Technology Innovation Fund (2014CXLG11),Tianjin Key Laboratory of Marine Resources and Chemistry (201404),Yangtze Scholars and Innovative Research Team in University (IRT-17R81),Innovative Research Team of Tianjin Municipal Education Commission (TD13-5008).
 Chinese Journal of Chemical Engineering2021年6期
Chinese Journal of Chemical Engineering2021年6期
- Chinese Journal of Chemical Engineering的其它文章
- Functional monodisperse microspheres fabricated by solvothermal precipitation co-polymerization
- Synthesized graphene oxide and fumed aerosil 380 dispersion stability and characterization with partially hydrolyzed polyacrylamide
- Synthesis and characterization of caprolactone based polyurethane with degradable and antifouling performance
- Removal of lead (Pb(II)) and zinc (Zn(II)) from aqueous solution using coal fly ash (CFA) as a dual-sites adsorbent
- Catalytic performance improvement of volatile organic compounds oxidation over MnOx and GdMnO3 composite oxides from spent lithium-ion batteries:Effect of acid treatment
- Application of fracturing technology to increase gas production in low-permeability hydrate reservoir:A numerical study
