Entacapone promotes hippocampal neurogenesis in mice
Dae Young Yoo , Hyo Young Jung Woosuk Kim , Kyu Ri Hahn Hyun Jung Kwon,Sung Min Nam, Jin Young Chung, Yeo Sung Yoon Dae Won Kim, In Koo Hwang
Abstract Entacapone, a catechol-O-methyltransferase inhibitor, can strengthen the therapeutic effects of levodopa on the treatment of Parkinson’s disease. However, few studies are reported on whether entacapone can affect hippocampal neurogenesis in mice. To investigate the effects of entacapone, a modulator of dopamine, on proliferating cells and immature neurons in the mouse hippocampal dentate gyrus, 60 mice (7 weeks old) were randomly divided into a vehicle-treated group and the groups treated with 10, 50, or 200 mg/kg entacapone. The results showed that 50 and 200 mg/kg entacapone increased the exploration time for novel object recognition. Immunohistochemical staining results revealed that after entacapone treatment, the numbers of Ki67-positive proliferating cells, doublecortin-positive immature neurons,and phosphorylated cAMP response element-binding protein (pCREB)-positive cells were significantly increased. Western blot analysis results revealed that treatment with tyrosine kinase receptor B (TrkB) receptor antagonist significantly decreased the exploration time for novel object recognition and inhibited the expression of phosphorylated TrkB and brain-derived neurotrophic factor (BDNF). Entacapone treatment antagonized the effects of TrkB receptor antagonist. These results suggest that entacapone treatment promoted hippocampal neurogenesis and improved memory function through activating the BDNF-TrkB-pCREB pathway. This study was approved by the Institutional Animal Care and Use Committee of Seoul National University (approval No. SNU-130730-1) on February 24, 2014.
Key Words: brain-derived neurotrophic factor; entacapone; hippocampus; neurogenesis; neurotrophic factor; phosphorylated cAMP response element-binding protein; tyrosine kinase receptor B receptor Chinese Library Classification No. R453; R364; Q2
Introduction
Abnormal neuronal loss due to trauma and pathological factors can lead to neurological diseases and functional impairment (Eriksson et al., 1998). The hippocampus plays important roles in learning and memory, spatial cognition and short-term memory to long-term memory conversion(Burgess et al., 1999; Deng et al., 2010). Moreover, the hippocampus is the most susceptible brain region to damage induced by Alzheimer’s disease and ischemia (Kappor et al., 2019; Beason-Held et al., 2020). In previous decades,the hippocampus was widely studied because new neurons emerge from the restricted regions of the central nervous system (CNS), such as the subventricular zone (SVZ) and the hippocampal subgranular zone (SGZ) (Eriksson et al., 1998;Kempermann and Gage, 2000; van Praag et al., 2002; Fares et al., 2019). In the hippocampus, neural stem cells located in the SGZ can divide and further migrate and integrate into the granule cell layer (van Praag et al., 2002). In humans,approximately 700 neurons are newly added into granule cells each day (Spalding et al., 2013). The surviving cells extend their axons, the mossy fibers, into the hippocampal CA3 region(van Praag et al., 2002). Electrophysiological, optogenetic,and retrograde tracing studies confirmed that glutamatergic synaptic responses or inputs in newly emerging neurons are detectable up to several weeks (Ge et al., 2006; Chancey et al., 2013; Deshpande et al., 2013).
Various intrinsic and extrinsic factors influence adult hippocampal neurogenesis (Niklison-Chirou et al., 2020).Monoamines and their modulators are presumed to possibly contribute to a hippocampal neurogenesis as they affect the hippocampal neurochemistry and behavioral responses (Park, 2019). We previously demonstrated that pyridoxine and pyridoxal 5′-phosphate, a cofactor in the synthesis of monoamine including dopamine, are crucial for neurogenesis and cognitive functions including memory(Jung et al., 2017, 2020). Dopamine is an important monoamine neurotransmitter, which regulates the mood,motivation, cognition, reward, and motor control (Ohira,2020). In addition, dopamine agonists induce the release of acetylcholine from the hippocampus (Imperato et al., 1993).Dopamine regulates cell proliferation, and thus mammalian brain development; dopamine and its receptors are closely related to hippocampal neurogenesis (Mishra et al., 2019a;Ohira, 2020).
Entacapone, a catechol-O-methyltransferase (COMT)inhibitor, is used to treat Parkinson’s disease together with levodopa (L-DOPA) (Liao et al., 2020). Entacapone inhibits COMT through degrading L-DOPA into 3-methoxy-4-hydroxy-L-phenylalanine, and consequently, it increases the plasma concentration of L-DOPA, which can cross the blood-brain barrier (Rinne et al., 1998). Entacapone prevents the effects of scopolamine and prolongs the retention latency in the passive avoidance test (Khromova et al., 1997); however, it has no significant effects on the cognition of Sprague-Dawley rats(Detrait et al., 2016).
Nevertheless, it is difficult to prove that COMT inhibitors influence neurogenesis in the mouse brain, and few reports suggest that dopaminergic transmission and dopamine receptors may be neuroprotective and may induce neurogenesis (Schlachetzki et al., 2016; Ermine et al., 2018;Mishra et al., 2019a, b). Therefore, in the present study,we investigated the effects of entacapone on hippocampal neurogenesis based on behavioral, morphological, and biochemical analysis in mice.
Materials and Methods
Experimental animals
Male 7-week-old C57BL/6J mice (22-25 g,n= 60) were obtained from Orient Bio (Sungnam, South Korea). Animals were housed in specific pathogen free facility in Seoul National University College of Veterinary Medicine under adequate temperature, humidity, and light/dark cycle. Experimental protocols for animal experiment were approved by the Institutional Animal Care and Use Committee of Seoul National University (approval No. SNU-130730-1) on February 24, 2014.
Entacapone and TrkB receptor antagonist treatment
After 1-week acclimation, mice (n= 10 per group) were randomly divided into four groups to identify the effects of entacapone on hippocampal function: the vehicle-treated control group and the groups treated with 10, 50, or 200 mg/kg entacapone (E10, E50, and E200 groups, respectively). To elucidate the effects of E50 and/or TrkB receptor antagonist on hippocampal function, mice (n= 5 per group) were divided into vehicle (artificial cerebrospinal fluid)-treated control group, TrkB receptor antagonist N-[2-[[(Hexahydro-2-oxo-1H-azepin-3-yl)amino]carbonyl]phenyl]benzo[b]thiophene-2-carboxamide (ANA-12, Sigma, St. Louis, MO, USA)-treated(ANA-12 alone) group, E50 alone group, and E50 + ANA-12 treatment (E50 + ANA-12) group. Vehicle or entacapone was orally administered to the mice once a day using a feeding needle and ANA-12 was injected into the hippocampus at a rate of 0.5 μL/min for 5 minutes according to the mouse atlas provided by Paxinos and Franklin (2001). We administered entacapone for 21 days because immature neurons transiently express doublecortin (DCX) only from 1 day to 28 days after birth (Brown et al., 2003; Couillard-Despres et al., 2005).
Memory testTo investigate the effects of entacapone and/or TrkB receptor antagonist on hippocampus-dependent memory, the novel object recognition (NOR) test was conducted using a black acryl box (25 cm× 25 cm × 25 cm), as per a previously described method (Jung et al., 2017). Briefly, the mice explored the open box for 2 minutes on day 20 of entacapone treatment to adapt to the environment. On day 21, the two same objects were put in the opposite corners of the box and mice were allowed to explore the two objects for 2 minutes,following which the mice were removed from the apparatus.One hour after the training trial, the mice were again allowed to explore the familiar and new objects. Exploration time was determined when the noses of the mice were about 2 cm away from the objects.
The difference between the familiar and new objects on the testing day (day 21) was calculated as the discrimination index (DI) by evaluating the proportion of time difference in observing the new and familiar objects versus total explorationtime on the two objects during the test trial.
Immunohistochemistry
To observe the effects of entacapone on the proliferating cells, differentiated neuroblasts, and phosphorylation of cAMP response element binding protein at Ser133 in the hippocampal dentate gyrus, immunohistochemical staining was performed for Ki67, DCX, and the phosphorylated cAMP response element binding protein (pCREB), respectively.Briefly, mice (n= 5 per group) were anesthetized with intraperitoneal injection of alfaxalone (Alfaxan, 75 mg/kg;Careside, Seongnam, South Korea) and xylazine (10 mg/kg;Bayer Korea, Seoul, South Korea) 2 hours after NOR test and were perfused transcardially (Jung et al., 2017). Mouse brain was serially cut into 30 μm-thick sections between 1.7 mm and 2.3 mm posterior to the bregma, according to the mouse atlas provided by Paxinos and Franklin (2001), and the sections were collected into six-well plates.
Five sections 90 μm apart were selected and used for immunohistochemical staining, as described in a previous study (Yoo et al., 2019). Briefly, sections were sequentially incubated with 5% normal goat serum (Vector Lab.,Burlingame, CA, USA) and rabbit anti-Ki67 antibody (1:1000,Cat# ab15580, Abcam, Cambridge, UK), rabbit anti-DCX antibody (1:5000, Cat# ab18723, Abcam), or rabbit anti-pCREB(1:400, Cat# 9198, Cell Signaling Technology Inc., Beverly,MA, USA) for 12 hours at 25°C. Thereafter, sections were incubated with goat anti-rabbit IgG (Cat# BA-1000, Vector Lab.) and Vectastain ABC kits (Cat# PK-6100, Vector Lab.).Immunoreaction was visualized using 3,3′-diaminobenzidine tetrachloride (Sigma) in 0.1 M Tris-HCl buffer (pH 7.2) and the immunoreactive structures were taken with a BX51 light microscope (Olympus, Tokyo, Japan) equipped with a digital camera (DP72, Olympus).
For each antibody, five sections 90 μm apart from each other were examined between 1.7 mm and 2.3 mm posterior to the bregma according to the mouse atlas by Paxinos and Franklin(2001) and DCX immunoreactivity was measured as described previously (Yoo et al., 2019). Briefly, DCX-immunoreactive structures in the dentate gyrus were photographed and the optical density (OD) was measured using ImageJ software v.1.5 (National Institutes of Health, Bethesda, MD, USA). DCX immunoreactivity was calculated by summation of OD × pixel numbers and final data were expressed as a percentage of the control group values (set to 100%).
The number of Ki67- and pCREB-positive cells in the dentate gyrus was counted with Optimas 6.5 software (CyberMetrics,Scottsdale, AZ, USA) according to a previously described method (Yoo et al., 2019). Cell counts from all the sections (n= 5) of each mouse (n= 5) were averaged.
Western blot analyses
To investigate the effects of entacapone and/or TrkB receptor antagonist on expression levels of brain-derived neurotrophic factor (BDNF) and tyrosine kinase receptor B (TrkB) in the hippocampus, mice (n= 5 per group) were euthanized with a mixture of alfaxalone and xylazine 2 hours after NOR test. Hippocampal tissues were quickly removed from the whole brain and were homogenized in a buffer,as described previously (Jung et al., 2017). Briefly, proteintransferred nitrocellulose membranes (Pall Crop, East Hills,NY, USA) were treated with a mature form of rabbit anti-BDNF(1:5000, Cat# ab108319, Abcam), rabbit anti-phosphorylated TrkB (1:500, Cat# sc-135645, Santa Cruz Biotechnology,Santa Cruz, CA, USA), and rabbit anti-β-actin (1:1000, Cat#8457, Cell Signaling) for 12 hours at 4°C. Thereafter, the membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1:500, Cat# PI-1000, Vector). The proteins were detected using enhanced chemiluminescent reagent(Amersham, Buckinghamshire, UK) protocol according to the manufacturer’s instructions. Data were normalized compared with the β-actin levels as demonstrated in the previous study(Jung et al., 2017). Quantification of the bands was analyzed using ImageJ software v. 1.50 (National Institutes of Health).
Statistical analysis
Data represent the mean ± SD. Raw data were statistically analyzed using one-way analysis of variance followed by Tukey’s multiple-range test using GraphPad Prism 5.01 software (GraphPad Software Inc., La Jolla, CA, USA). Data were considered significant atP< 0.05.
Results
Effects of entacapone on memory measured by NOR test
Animals in each group revealed no significant difference during exploration of the two identical objects during the training trial. During the test trial, however, the vehicletreated (control) and 10 mg/kg, 50 mg/kg, and 200 mg/kg entacapone-treated (E10, E50, and E200) mice spent moretime with the new object than that with the familiar object (P< 0.05). Particularly, DI was significantly increased in the E50 and E200 groups compared with that in the control and E10 groups (P< 0.05; Figure 1).
Most of the Ki67-positive proliferating cells were observed in the SGZ of the dentate gyrus (Figure 2A). In total, 21 and 23 Ki67-positive cells per section were detected in the control and E10 groups (Figure 2E), respectively, and there was no significant difference between the control and E10 groups(Figure 2B and E). In the E50 and E200 groups, entacapone treatment significantly increased the mean number of Ki67-positive cells compared with that in the control and E10 groups (P< 0.05; Figure 2C-E). Moreover, 33.0 and 33.8 Ki67-positive cells per section were observed in the E50 and E200 groups, respectively (Figure 2E).
Effects of entacapone on DCX-positive differentiated neuroblasts
In the control group, the cell bodies of the DCX-positive neuroblasts were detected in the SGZ of the dentate gyrus and they stretched their dendritic branches into the molecular layer (Figure 3A). There was no significant difference in DCX immunoreactivity between the control and E10 groups (Figure 3B and E). DCX immunoreactivity in the E50 group was 180.6%of that in the control group;P< 0.05; Figure 3C and E). DCX immunoreactivity in the E200 group was decreased compared with that in the E50 group; however, DCX immunoreactivity in the E200 group was significantly higher than that in the control group (P< 0.05; Figure 3D and E).
Effects of entacapone on phosphorylation of CREB signaling
In the control group, pCREB immunoreactivity was clearly detected in the nuclei located in the molecular layer and SGZ of the hippocampal dentate gyrus (Figure 4A). In the E10 group, pCREB-immunoreactive nuclei were hardly detected in the SGZ of the dentate gyrus compared with those in the control group, although no significant difference was observed in the number of the pCREB-positive nuclei (Figure 4B and E). In the E50 and E200 groups, more abundant pCREBpositive nuclei were detected in the SGZ of the dentate gyrus compared with those in the control group (P< 0.05; Figure 4C and D), and the count of pCREB-positive nuclei was 138.2%and 136.0% of that in the control group, respectively (Figure 4E).
Effects of entacapone on BDNF-TrkB signaling pathway
Western blot analysis for mature BDNF and phosphorylated TrkB was performed to examine the role of entacapone in the BDNF-TrkB signaling pathway. In the E10, E50, and E200 groups, the levels of BDNF and TrkB were significantly increased compared with those in the control group (P<0.05). Mean percentages of relative optical density (ROD) in mature BDNF were 169.0%, 162.3%, and 194.5%, respectively,compared with 100% in the control group; whereas those in TrkB were 151.1%, 156.0%, and 168.2%, respectively,compared with 100% in the control group (Figure 5).
Effects of TrkB receptor antagonist on memory measured by NOR test
Hi! My name is Ashley. When I met Bryan during the last year in college it was ugh at first sight. I thought he was the good-looking jock and acted kind of cocky. It only made him charming. How I eventually found out he thought I was a suck-up because I was focused on studying and wanted to graduate as an A student. He practically avoided me wherever I showed up and changed directions if he would see me from far away.
During training trial, animals in the ANA-12 alone group spent less, but not significant, time compared with other groups in exploring the two identical objects. During test trials, mice spent more time to explore new object than to find familiar one in control, E50 alone and E50 + ANA-12 groups (P< 0.05).However, in the ANA-12 alone group, mice spent similartime to explore new and familiar objects. In addition, DI was significantly decreased in the ANA-12 alone group compared with that in the control or E50 alone group (P< 0.05) and was recovered in E50 + ANA-12 group to similar levels to those in the control group (Figure 6).
Effects of TrkB receptor antagonist on BDNF-TrkB signaling pathway
Mature BDNF and phosphorylated TrkB levels were measured to confirm the TrkB inhibition and BDNF-TrkB signaling of entacapone. In the ANA-12 alone group, BDNF and TrkB levels were significantly decreased to 53.0% and 34.7% of control group, respectively. In the E50 alone group, BDNF and TrkB levels were significantly increased to 171.1% and 161.0% of those in the control group, respectively (P< 0.05). In the E50 +ANA-12 group, BDNF and TrkB levels in the hippocampus were 153.4% and 154.4% of those in the ANA-12 alone group (P<0.05; Figure 7).
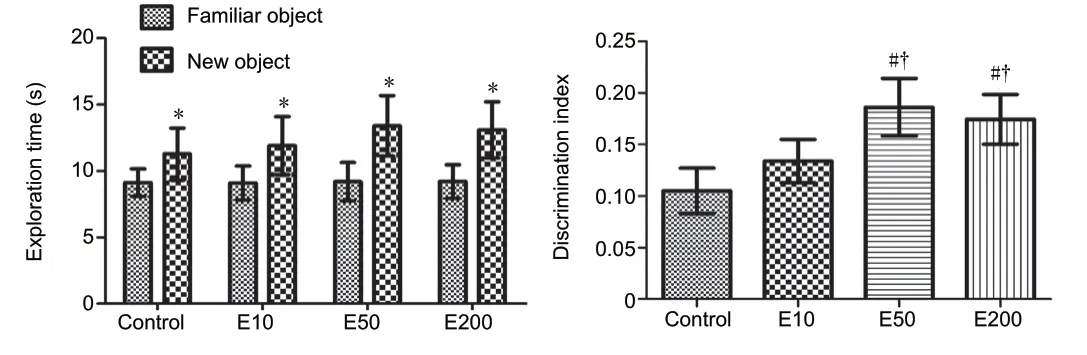
Figure 1 |Exploration time (familiar and new object) and the discrimination index (the test trial) in the novel object recognition test in the vehicle-treated (control) and 10, 50, and 200 mg/kg entacaponetreated (E10, E50, and E200) mice.

Figure 2 |Immunohistochemistry for Ki67 in the mouse dentate gyrus of the vehicletreated (control, A) and 10 (B), 50 (C), and 200 mg/kg (D) entacapone-treated (E10,E50, and E200) groups.
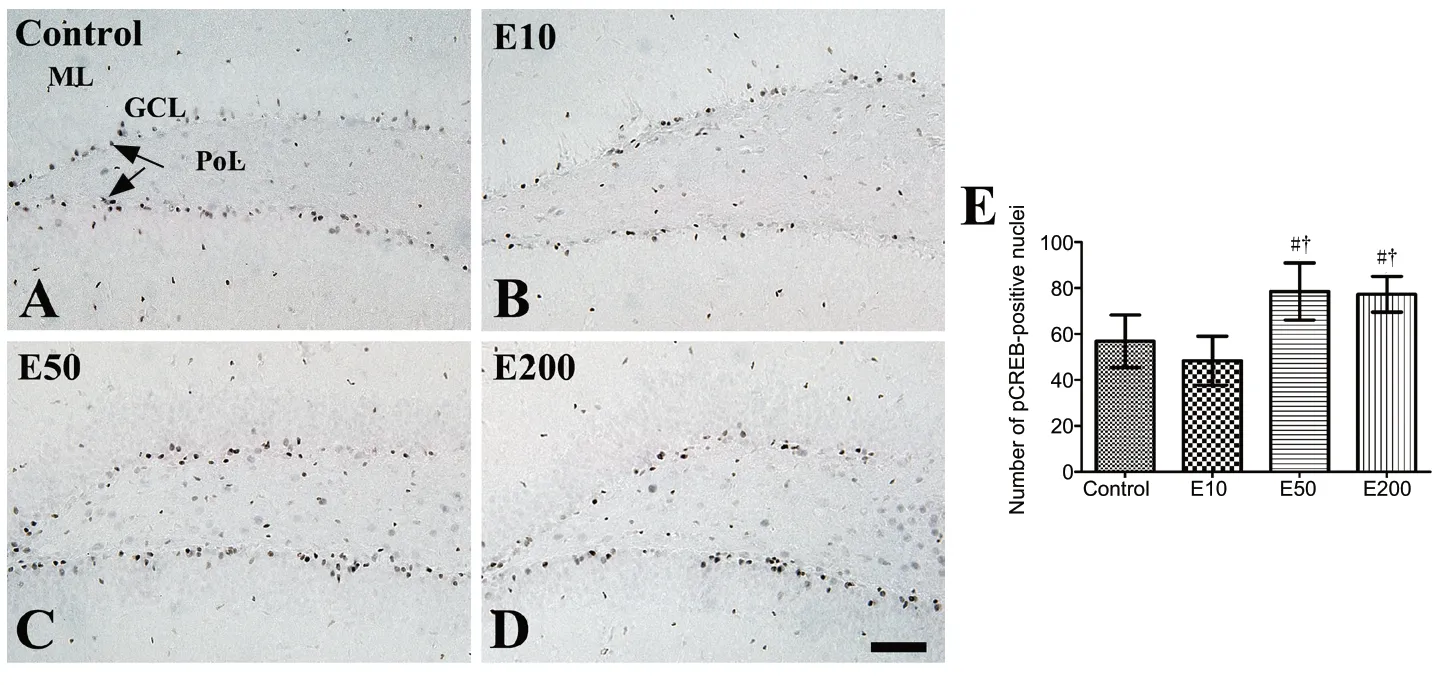
Figure 4 |Immunohistochemistry for phosphorylated cAMP response elementbinding protein in the mouse dentate gyrus of the vehicle-treated (control, A) and 10(B), 50 (C), and 200 mg/kg (D) entacaponetreated (E10, E50, and E200) groups.
Discussion
Entacapone, a COMT inhibitor, directly reg ulates L-DOPA metabolism (Rinne et al., 1998). Dopamine is released in various regions of the mammalian brain, and along with its receptors, it modulates the proliferation of neural stem cells, and thus, the early embryonic development of the nervous system as well as adult hippocampal neurogenesis(Mishra et al., 2019a; Ohira, 2020). The adult brain comprises two neurogenic regions, which contain adult neural stem/progenitor cells, and the neurogenic proliferation may be regulated by dopamine (Schlachetzki et al., 2016; Vargas-Saturno and Ayala-Grosso, 2018). Moreover, dopamine depletion or lesioning reduces hippocampal neurogenesis in rat and mouse models of Parkinson’s disease (Schlachetzki et al., 2016; Ermine et al., 2018; Mishra et al., 2018). In this study, we examined the effects of entacapone on hippocampus-dependent memory, proliferating cells, and differentiated neuroblasts using NOR test, Ki67, and DCX immunostaining, respectively.
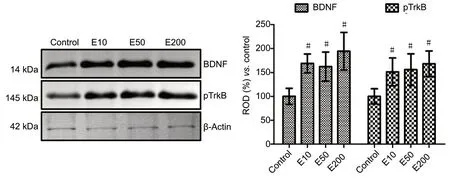
Figure 5 |Western blot analysis of the BDNF and pTrkB in the hippocampi of the vehicle-treated (control) and 10, 50, and 200 mg/kg entacaponetreated (E10, E50, and E200) mice.
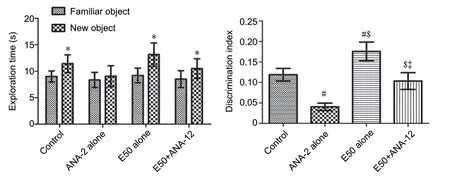
Figure 6 |Object exploring time in training and testing trials and the discrimination index in the testing trial in the vehicle-treated (control)group, 2.5 μL TrkB receptor antagonist ANA-12-treated (ANA-12 alone)group, 50 mg/kg entacapone-treated (E50 alone) group, and E50 + ANA-12 treatment (E50 + ANA-12) group.
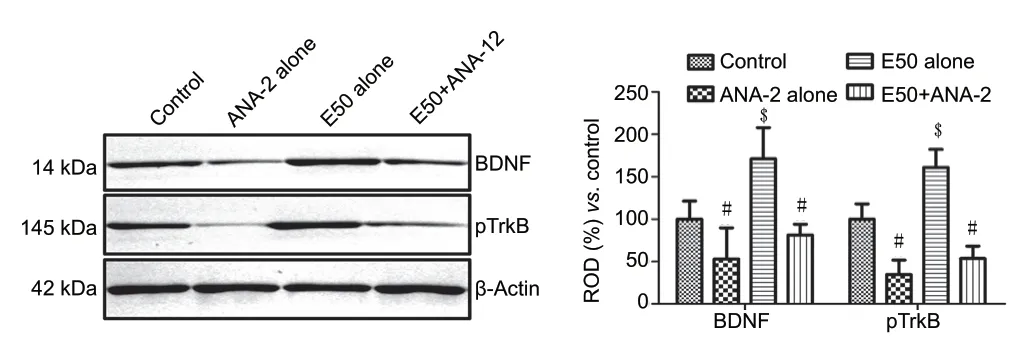
Figure 7 |Western blot analysis of the mature form of BDNF and pTrkB in the vehicle-treated (control) group, 2.5 μL TrkB receptor antagonist ANA-12-treated (ANA-12 alone) group, 50 mg/kg entacapone-treated (E50 alone) group, and E50 + ANA-12 (E50 + ANA-12) group.
Entacapone treatment increased the exploration time on new objects, although there was no significant difference in the time taken for exploring new and familiar objects.However, DI was significantly increased in the E50 and E200 groups compared with that in the control or E10 group. A previous study demonstrated that treatment with 30 mg/kg entacapone led to a moderate increase in the cognition index in Sprague-Dawley rats, and no significant difference was observed (Detrait et al., 2016). Recent studies also reported that spatial learning and memory were closely related to the dopamine release in the axons of dorsal hippocampus from neurons in the locus coeruleus (Kempadoo et al., 2016;Yamasaki and Takeuchi, 2017). Administration of entacapone significantly increased the number of Ki67-positive nuclei and DCX-positive neuroblasts in the hippocampal dentate gyrus of mice. Dopamine and its receptors are crucial in the dopaminedependent neurogenesis in the SGZ and SVZ (Winner et al.,2006; O’Keeffe et al., 2009; Mishra et al., 2019a; Shuto et al.,2020). Neural precursor cells express both dopaminergic D1-and D2-like receptors, but only the activation of D1 receptor improves hippocampal neurogenesis (Mishra et al., 2019b).Dopaminergic denervation or stimulation influence the proliferation not only in the SVZ, but also in the hippocampus(Winner et al., 2006; Park and Enikolopov, 2010; Hedlund et al., 2016; Tapia-Bustos et al., 2017).
Neurotrophins including BDNF modulate the cell proliferation and differentiation of neural stem cells in several neurogenic regions of the mammalian brain (Pencea et al., 2001; Rossi et al., 2006; Cruz et al., 2018). In particular, BDNF and its receptor, TrkB, play a pivotal role in neurogenesis and synaptic plasticity, and the signaling pathways mediated by these molecules are essential in regulating cell proliferation and survival in the hippocampus (Rossi et al., 2006). The expression levels of BDNF and TrkB in the hippocampus are controlled via extrinsic stimulating factors (Gustafsson et al., 2003). Küppers and Beyer (2001) demonstrated that a dopaminergic activity can modulate the release and expression of BDNFin vitro. In addition, local infusion of BDNF increased the expression of dopaminergic D3 receptors(Guillin et al., 2001; Pencea et al., 2001), and the stimulation of dopaminergic D1 receptor markedly increased the dopaminergic neurogenesis in 6-hydroxydopamine lesioned rats (Mishra et al., 2019a). In the present study, we confirmed that treatment of entacapone significantly increased BDNF and phosphorylated TrkB expression levels in the hippocampal homogenates. Moreover, the expression of pCREB, which is a nuclear effector of BDNF-TrkB signaling, was significantly enhanced. However, in the present study, we observed the significant increases of BDNF and TrkB expression in the hippocampus with 10 mg/kg entacapone treatment, while we did not observe any significant changes in the memory measured by NOR test as well as Ki67-, DCX-, and pCREBpositive cells in the dentate gyrus. This result suggests that 10 mg/kg entacapone significantly increase BDNF and phosphorylated TrkB expression in the hippocampus because BDNF, an effector of immediately early gene, is rapidly induced without intervening protein synthesis (Paolantoni et al., 2018).
The BDNF-TrkB signaling induces the phosphorylation of CREB,which regulates synaptic plasticity and memory formation(Silva et al., 1998; Foltran and Diaz, 2016; Zagrebelsky et al.,2020), and the phosphorylation of CREB was prevented by an anti-BDNF antibody treatment (Simonetti et al., 2008). In the present study, the treatment with ANA-12, a TrkB receptor antagonist, significantly decreased the NOR memory and BDNF and TrkB expression in the hippocampus. Treatment with entacapone significantly ameliorates the reduction of NOR memory and decreases in BDNF and TrkB expression in the hippocampus. Previous studies have demonstrated that treatment with ANA-12 shows significant impairment in memory retention in rats (Blank et al., 2016) and blocked the enhanced synaptic plasticity and memory improvement induced by environmental enrichment in nerve-injured mice(Wang et al., 2019).
It has been reported that dopaminergic transmission from the midbrain enhances long-term potentiation (LTP) in the hippocampus by activating the D1-like receptors (Li et al., 2003; Lisman and Grace, 2005; Lemon and Manahan-Vaughan, 2006). LTP induced in the dentate gyrus increases cell proliferation and neuronal survival of the newly generated cells (Bruel-Jungerman et al., 2006). In addition, the CREBdependent transcription enhances hippocampal latephase LTP (Gruart et al., 2012), and we confirmed that the modulation of dopamine signaling by entacapone increased pCREB expression in the mouse hippocampus. Activation of the dopaminergic inputs enhances the hippocampal synaptic plasticity by promoting an LTP formation, and thus a hippocampus-dependent memory (Lisman and Grace, 2005).Furthermore, the dopaminergic loop in the ventral tegmental area and hippocampus is crucial for the formation of longterm memory traces (Lemon and Manahan-Vaughan, 2006).
In the present study, we demonstrated the possible mechanisms of entacapone on the NOR memory, proliferating cells and neuroblasts in the dentate gyrus. However, we did not observe entacapone-enhanced integration of neuroblasts into fully mature neurons in the dentate gyrus. In addition,proteomics or microassay studies are needed to investigate the effects of entacapone on hippocampal function.
In conclusion, administration of entacapone influences NOR memory and hippocampal neurogenesis by changing the BDNF-TrkB-pCREB pathway in the mouse hippocampus.
Author contributions:All authors conceived the study and manuscript. DYYand IKH designed the experiment. DYY wrote the manuscript and IKH supervised all experiments. DYY, HYJ, WK, and KRH conducted the immunohistochemistry and analyzed the data. HJK and DWK did the western blot study. SMN, JYC,and YSY advised the design of the study and edited the manuscript. All authors approved the final version of this paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP)(NRF-2016R1A2B4009156), the Promising-Pioneering Researcher Program through Seoul National University (SNU) in 2015 and by the Research Institute for Veterinary Science, Seoul National University.
Institutional review board statement:The study was approved by theInstitutional Animal Care and Use Committee of Seoul National University(approval No. SNU-130730-1) on February 24, 2014.
Copyright license agreement:The Copyright License Agreement has beensigned by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation
- Normobaric oxygen therapy attenuates hyperglycolysis in ischemic stroke
- MicroRNA-670 aggravates cerebral ischemia/reperfusion injury via the Yap pathway
- Corticospinal excitability during motor imagery is diminished by continuous repetition-induced fatigue
- TP53-induced glycolysis and apoptosis regulator alleviates hypoxia/ischemia-induced microglial pyroptosis and ischemic brain damage
- Apelin-13 inhibits apoptosis and excessive autophagy in cerebral ischemia/reperfusion injury