Vascular inflammation in the central nervous system
Xinying Guo, Zhen Zhao
Vasculature is the interface between tissue and circulation. It consists of endothelial cells, mural cells including vascular smooth muscle cells and pericytes, and other perivascular cells including macrophages and fibroblasts (Sweeney et al., 2019).The vascular system not only delivers oxygen and nutrients, but also shuttles the immune cells around. As the first line of defense, the vascular system also senses the changes in surrounding tissue, particularly inflammation. Vascular inflammation can occur in blood vessels of all sizes in any organ. It has a complex etiology, including infections such as coronavirus disease-19(COVID-19), and chronic conditions such as diabetes, hypertension and neurodegenerative diseases (Hanafi et al.,2020). Excessive vascular inflammation is clinically known as vasculitis, diagnosed by blood test, imaging and biopsy. Vasculitis not only thickens the blood vessel wall, causing blood flow reduction and insufficient delivery of oxygen and nutrients, but also triggers inflammatory responses in secondary sites,or even the whole body.
Vascular inflammation in central nervous system (CNS) diseases: Inflammation of the CNS is a common feature of many neurodegenerative diseases.Neuroinflammation, where microglia and astrocytes are overactivated, often fosters a dysfunction of neurovascular unit and exacerbates neuronal loss. On the other hand, blood vessels are another important site of CNS inflammation.Vascular inflammation is characterized by the presence of activated endothelial cells. These activated endothelial cells upregulate receptors necessary for leukocyte recruitment, making the vessel “sticky” to immune cells and allowing for extravasation of peripheral immune cells into the brain parenchyma (Figure 1). Therefore, vasculitis is often associated with neurological complications, including headache,brain edema, stroke, and even dementia(Birnbaum and Hellmann, 2009).
The brain vasculature is perhaps a more significant target of inflammation because of the presence of the blood-brain barrier(BBB). During vascular inflammation,activated endothelial cells display an altered endophenotype and a decrease in tight junction proteins, impairing BBB functions and allowing the entry of peripheral immune cells and toxic/inflammatory plasma products into the parenchyma (Figure 1).Although BBB dysfunction is now widely accepted as one of the major driving forces in neurodegenerative diseases including multiple sclerosis, Parkinson’s disease and Alzheimer’s disease (AD), and brain injuries such as stroke, epilepsy and traumatic brain injury (Sweeney et al., 2019), our understanding of vascular inflammation remains limited.
It remains largely unknown how systemic vascular inflammation activates the innate immune cells of the CNS and triggers neuronal impairment, or whether vascular inflammation also influences the pathological cascades in age-associated neurodegenerative diseases, such as misfolded protein aggregation in AD.However, molecular signatures of vascular inflammation and endothelial activation have been shown to contribute to the harmful circulatory environment in aging and AD.This might be just the tip of the iceberg, as other vascular cells are yet to be studied.
Role of pericytes in CNS vascular inflammation: Brain pericytes are located perivascularly between the endothelium and parenchyma, and are implicated in a range of neurological disorders including AD and ischemic stroke (Sweeney et al.,2019). Besides maintaining BBB functions and clearance of waste products (Sweeney et al., 2019), they are also situated ideally to control several aspects of the CNS immune responses, including leukocyte extravasation,propagation of peripheral and central inflammation, polarization of inflammatory cells in the perivascular space, and even adaptive immunity regulation.
Studies in the pericyte-deficientPdgfbret/retmouse model have mapped the proinflammatory endothelial changes in the absence of pericytes(M?e et al., 2021).At single-cell transcriptomic level, several pro-inflammatory mediators, such as angiopoietin-2 (Angpt2), intercellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1),were dramatically upregulated in brain endothelial cells after pericytes loss (M?e et al., 2021). Angiopoietin-2 is required for claudin 5-containing tight junction in the brain microvessels, and Angpt2 knockout mice exhibit BBB disruption and vascular malformation (M?e et al., 2021).However, deregulated signaling between Angiopoietin-2 and its cognate endothelial Tie2 receptor often contributes to the aggravated neuroinflammation; therefore, in the absence of pericytes, endothelial cells try to compensate with its own Angiopoietin-2 at the expanse of vascular inflammation.In addition, brain pericytes are important to limit leukocyte infiltration into the CNS during homeostasis and autoimmune neuroinflammation (T?r?k et al., 2021).The upregulation of leukocyte adhesion molecules in the adult vasculature of pericyte deficient mice (T?r?k et al., 2021),including VCAM-1 and ICAM-1, is often accompanied by increased leukocyte entry into the brain parenchyma.
Pericyte injury and loss are quite common in CNS injuries including ischemia, hypoxia and traumatic brain injury (Wu et al., 2021),and neurodegenerative diseases including but not limited to AD, Parkinson’s disease,multiple sclerosis, amyotrophic lateral sclerosis, cerebral small vessel diseases(Sweeney et al., 2019) (Figure 1). As the permissiveness of the vasculature toward leukocyte infiltration is highly related to the loss of vessel pericytes coverage (T?r?k et al., 2021), it suggests that pericyte loss in head injuries such as traumatic brain injury(Wu et al., 2021) may also trigger vascular inflammation.
Pericyte involvement in infection and neurological complications: As coronavirus disease 2019 (COVID-19) is spreading rapidly in all countries, the conspicuous vascular complications and neurological manifestations have emerged worldwide in COVID-19 patients. There is a broad spectrum of symptoms, including anosmia and ageusia in early and mild conditions, myopathy in peripheral, and impaired consciousness in severe cases likely due to stroke, venous thromboembolism, intraventricular and subarachnoid hemorrhage, encephalitis and seizures, suggesting a complex interplay between infection, systemic immune response and cerebrovascular impairment(Figure 2). SARS-CoV-2 virus uses the spike glycoprotein to find its entry receptor angiotensin-converting enzyme 2 (ACE2).Interestingly, even though airway epithelial cells are the major ACE2 expression site in the lung, pericytes in other organs such as brain and heart are also enriched in ACE2 expression (He et al., 2020), indicating they could be targets of the virus once it spreads to the circulation (Figure 2). In addition, severe COVID-19 patients manifest acute respiratory distress syndrome, with pulmonary pericyte loss in alveolar capillaries(Cardot-Leccia et al., 2020), suggesting that SARS-CoV-2 infection may render pericyte injury. Pericyte loss promotes endothelial production and release of Von Willebrand factor (M?e et al., 2021), which induces platelet aggregation, blood coagulation and leukocyte recruitment (Kawecki et al.,2017) (Figure 1). Therefore pericyte injury in COVID-19 patients contributes to activation of pro-inflammatory and pro-thrombotic responses in the neighboring endothelial cells, and the vascular inflammation and microangiopathy in the severe cases(Ackermann et al., 2020).
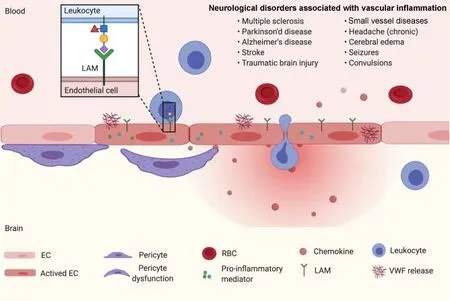
Figure 1 |Role of pericytes in vascular inflammation.The absence of pericytes and/or pericyte dysfunction has been found in many neuroinflammatory diseases. The upregulation of several pro-inflammatory mediators, such as angiopoietin-2 (Angpt2),production and release of Von Willebrand factor, and leukocyte adhesion molecules, such as intercellular adhesion molecule 1 and vascular adhesion molecule 1, in endothelial cells after pericytes loss. The proinflammatory phenotype of the brain endothelium of pericyte loss substantially contributes to the massive leukocyte infiltration during neuroinflammation. In addition, blood-brain barrier breakdown,one of the pathological hallmarks of neuroinflammatory diseases, is an early even in the formation of the inflammatory lesions and has been suggested to precede parenchymal inflammation. EC: Endothelial cell; LAM: leukocyte adhesion molecules; RBC: red blood cell; VWF: Von Willebrand factor.
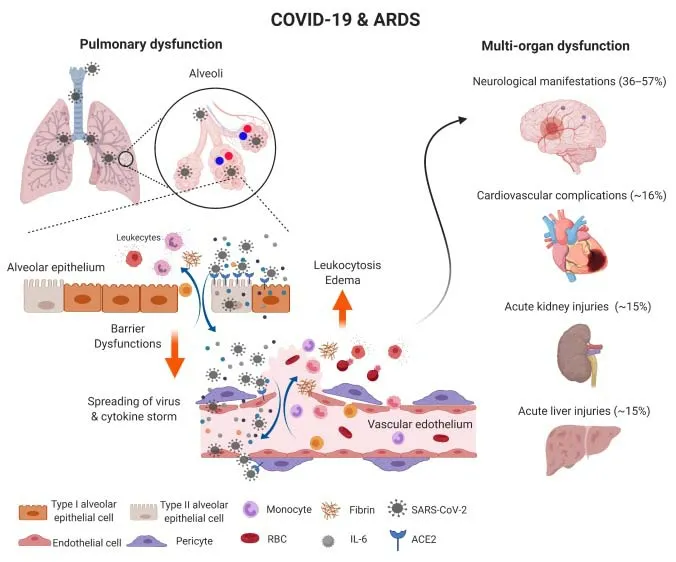
Figure 2 |Microvascular inflammation in COVID-19.SARS-CoV-2 infection in the lower respiratory system favors the alveolar epithelial cells that express ACE2, which leads to alveoli inflammation, excessive production of chemokine and cytokine productions,oxidative stress, pyroptosis and breakdown of epithelial barrier. SARS-CoV-2 and inflammatory molecules attack pulmonary microvessels close by, damage both endothelial cells and pericytes, and breach the blood-lung barrier. Through the circulation, SARS-CoV-2 and cytokine storm will quickly reach multiple organs, breakdown blood-brain barrier, blood-heart barrier, blood-liver barrier and blood-kidney barrier,which not only cause vascular inflammation (vasculitis) and perivascular necrosis and tissue damage,but also result in clogging and vascular diseases including thrombosis, cardiac arrest, stroke, etc. ACE2:Angiotensin-converting enzyme 2; COVID-19: coronavirus disease-19; IL-6: interleukin 6; RBC: red blood cell; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
In fact, pericytes are broadly targeted by human viruses, including but not limited to HIV-1, cytomegalovirus, zika and Japanese encephalitis virus. Pericytes are also highly enriched with Aminopeptidase N (also known as CD13), an entry receptor for most alphacoronaviruses, including HCoV-229E, suggesting pericyte is especially susceptible to coronavirus and might be commonly involved in infection-related vascular complications. As the pericyte is the gatekeeper of the blood-brain barrier and plays an important role in cerebrovascular and neurodegenerative diseases (Sweeney et al., 2019), more attentions should be paid to this otherwise forgotten cell type in COVID-19, and its potentially link to the neurological manifestations in patients.
Vascular inflammation as a potential CNS therapeutic target: Vascular inflammation is present in almost all neurological diseases, and its attenuation is likely to be beneficial for BBB and neural protection.Due to the active communication with brain vasculature, pericytes represent an attractive CNS therapeutic target for the treatment of a diverse range of neurological disorders. Augmenting the anti-inflammatory function of pericytes or targeting specific factors in pericytes may attenuate vascular inflammation; and several attempts have been made to specifically target brain pericytes for therapeutic purpose. For example, administration of exogenous PDGF-BB is found to be neuroprotective in Parkinson’s disease and restoring BBB function in mice (Padel et al.,2016), which is consistent with PDGF-BB/PDGFR-β signaling as an essential pathway for the formation and maturation of BBB through the recruitment of pericytes.The upregulation of leukocyte adhesion molecules are also important steps of the cascade of leukocyte transmigration, leading to vascular inflammation. Accordingly, the treatment of a mix of anti-VCAM-1 and anti-ICAM-1 in pericyte-deficient mice has been found to ameliorate experimental autoimmune encephalomyelitis and reduce the number of infiltrated leukocytes (T?r?k et al., 2021). In addition, transplantation of pericytes derived from human pluripotent stem cells can efficiently reconstruct the BBB integrity and prevent neuronal apoptosis in a murine model of transient middle cerebral artery occlusion (Sun et al., 2020). These strategies are translatable for targeting pericyte dysfunction and associated vascular inflammation in CNS diseases.
However, two major issues should be addressed: how to get the drug across the BBB and how to make it act specifically on CNS pericytes. Firstly, unlike other cells in the brain parenchyma, pericytes are tightly connected with endothelial cells though peg-socket junctions, which means that they do not require a significant amount of drug diffusion once it passes the endothelial barrier. This also makes pericytes an attractive candidate for drug development than other perivascular cells. Secondly,given that pericytes are not specific to the brain but are present in the peripheral vasculature, targeting the CNS pericytes is much more complicated and challenging.The emerging technologies led by singlecell transcriptomics have provided new directions in this regard.
Conclusion and further direction: In summary, many CNS diseases exhibit features of vascular inflammation, and in response to injury, infection or disease,brain vasculature generates inflammatory mediators, which in turn induce chemokines and proinflammatory adhesion molecules,and recruit circulating immune cells. With the advanced and continually improved technologies and mutli-omics approaches,new inflammatory and anti-inflammatory mediators in endothelial cells and pericytes will emerge. The single cell transcriptomes of brain vasculature have provided us a much better understanding of pericyte identity,and its potential immune functions based on inflammatory mediators, anti-inflammatory factors and transcription factors. The identification and characterization of these mediators and factors will further provide promising targets and new approaches for developing interventions and treatments for related CNS diseases. In addition, given that basal permeability is lower in nonfenestrated vascular beds in brain (Sweeney et al.,2019), understanding the heterogeneity of different vascular cell types in CNS and peripheral systems during homeostasis and inflammation will be an important step to decipher the mystery of vascular inflammation. More importantly, piling evidence has demonstrated CNS pericytes as a key regulator of the neurovascular unit and a unique interface between the peripheral immune system and CNS, thereby contributing to vascular inflammation and neurovascular dysfunctions in many neurodegeneration diseases. It is especially interesting to determine whether there is a neuroinflammatory pericyte signature or endophenotype linked to vascular inflammation or specific neurodegenerative conditions, and understanding how pericytes contribute to CNS inflammation and neurovascular unit dysfunction will provide novel insights into the pharmacological management of a range of CNS diseases.However, it is still a long journey to a complete understanding of pericytes biology and functions.
The present work was supported by the National Institutes of Health (NIH),Nos. R01AG061288, R03AG063287,R01NS110687, R21AG066090, and 1RF1NS122060, Bright Focus Foundation, No.A2019218S and U.S. Department of Defense grant No. W81XWH2010424 (to ZZ).
Xinying Guo*, Zhen Zhao*Center for Neurodegeneration and Regeneration,Zilkha Neurogenetic Institute and Department of Physiology and Neuroscience, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA (Guo X, Zhao Z)Neuroscience Graduate Program, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA (Zhao Z)
*Correspondence to: Xinying Guo, MD,sarah_guoxy@163.com; Zhen Zhao, PhD,zzhao@usc.edu.https://orcid.org/0000-0002-8428-5036(Xinying Guo)https://orcid.org/0000-0001-8967-5570(Zhen Zhao)
Date of submission: May 21, 2021
Date of decision: August 3, 2021
Date of acceptance: August 22, 2021
Date of web publication: January 7, 2022
https://doi.org/10.4103/1673-5374.332140
How to cite this article:Guo X, Zhao Z (2022)Vascular inflammation in the central nervous system. Neural Regen Res 17(8):1728-1730.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
?Article author(s) (unless otherwise stated in the text of the article) 2022. All rights reserved.No commercial use is permitted unless otherwise expressly granted.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Motor neuron replacement therapy for amyotrophic lateral sclerosis
- Calorie restriction or dietary restriction: how far they can protect the brain against neurodegenerative diseases?
- Monodelphis domestica: a new source of mammalian primary neurons in vitro
- Proper progression of neurogenesis relies on a defined pattern of SUMOmodified proteins
- Elucidating the pathological mechanisms of neurodegeneration in the lethal serpinopathy FENIB
- Glial cell line-derived neurotrophic factor in brain repair after focal ischemic stroke