Molecular regulatory mechanism of ferroptosis and its role in gastrointestinal oncology:Progress and updates
INTRODUCTION
Cell death is a basic life process that is pivotal to the development and homeostasis of multicellular organisms.Functionally,cell death can be categorized into accidental cell death (ACD) and regulated cell death (RCD).ACD refers to instantaneous and catastrophic cell death due to severe physical (
,high pressure,high temperature,and hypertonicity),chemical (
,drastic pH fluctuations),or mechanical (
,shear force) damage.In contrast,RCD is triggered
specific molecular mechanisms and can be modulated (delayed or accelerated)
pharmacologic or genetic interventions[1].RCD can be further categorized by onset mechanism as apoptosis,autophagic cell death,paraptosis,mitotic catastrophe,oncosis,pyroptosis,autoschizis,necroptosis,entosis,or ferroptosis[1,2].
Ferroptosis is iron-dependent,non-apoptotic cell death characterized by the accumulation of free iron and lipid reactive oxygen species (ROS)[3].Studies have shown that the free iron concentration in gastrointestinal (GI) tumor cells is higher than that of normal cells,and the survival of tumor cells is highly dependent on the abnormally activated antioxidant system[2,3].Additionally,in recent years,a large number of studies have shown that the activation of ferroptosis can lead to GI tumor cell death[1-4].Thus,regulating ferroptosis in tumor cells may become a new therapeutic approach for GI tumors.Therefore,ferroptosis has become a research hotspot.
So the decision was made and the children began to make a new outfit21 for their Baby Jesus -- a little leather vest out of some scraps22 and some cloth diapers. Best of all, Baby Jesus fit perfectly into the little crib, but since it wasn’t quite time for him to sleep there yet, he was laid carefully on a shelf in the hall closet to wait for Christmas eve.
Here,we summarize recent research progress on the mechanism of ferroptosis and its role in GI tumors to expand ideas on clinical tumor treatment.
31.Stately castle, the master of which was an ogre: In some versions of the tale, the cat finds a castle whose owner is away. He quickly outwits the guards and then gains admittance for the Marquis and his bride. The former owner inexplicably dies while away and never returns to claim his property. In other versions, the Marquis quickly buys property with the money he receives as a dowry. Return to place in story.
DISCOVERY AND CHARACTERISTICS OF FERROPTOSIS
In 2003,Dolma
[4] identified a new compound while screening for compounds with killing effects against tumor cells.The identified compound,erastin,which selectively kills tumor cells expressing RASV12 protein,a mutated form of RAS.However,the erastin-mediated killing mechanism is different from that of previously known compounds,
it does not cause nuclear morphological changes,DNA fragmentation,or caspase-3 activation,and its cell-killing process cannot be reversed by caspase inhibitors[2].Then Yang
[5] and Yagoda
[6] found that erastinmediated cell death is inhibited by iron chelators and is accompanied by elevated intracellular ROS levels.Additionally,both studies identified RAS-selective lethal(RSL) compounds,RSL and RSL3,which trigger this type of cell death[3].In 2012,Dixon
[3] named this type of cell death ferroptosis,which is iron-dependent,nonapoptotic cell death characterized by intracellular ROS accumulation.In 2018,the Nomenclature Committee on Cell Death defined ferroptosis as a form of glutathione peroxidase 4 (GPX4)-regulated RCD that is triggered by oxidative stress in the intracellular microenvironment and can be inhibited by iron chelators and lipophilic antioxidants[1].
Ferroptosis is a novel type of iron-dependent cell death with genetic,biochemical,and morphological features different from other forms of cell death including apoptosis,unregulated necrosis,and necroptosis[3].The ultra-micromorphological features of ferroptosis include cell membrane disruption and blebbing,mitochondrial shrinkage,increased mitochondrial bilayer density,reduced or absent mitochondrial cristae,outer mitochondrial membrane disruption,normal nuclear size,and the absence of chromatin condensation[7].The main biochemical characteristics of ferroptosis include iron and ROS accumulation,protein kinase activation,cystine/glutamate antiporter inhibition,reduced cystine uptake and glutathione (GSH)synthesis,and nicotinamide adenine dinucleotide phosphate (NADPH) oxidation[8].
Both increased iron uptake and decreased iron elimination can enhance the sensitivity of cells to oxidative damage and ferroptosis
the Fenton reaction.Supplementation with exogenous iron ions but not other divalent metal ions can accelerate erastin-induced ferroptosis[3].Cells with mutated RAS show significantly increased iron uptake and significantly decreased iron storage capacity following the onset of ferroptosis[23].The intracellular level of labile iron (Fe
ions) is also a key factor affecting lipid peroxidation and ferroptosis.Upon exposure to different ferroptosis inducers,the intracellular Fe
ion level increases and various transport proteins associated with iron metabolism (
,ferritin and TFR1) are rearranged after ferroptosis[21].Iron overload and ferroptosis can be inhibited by knocking out genes encoding transferrin receptors or upregulating the expression of iron-storage proteins.Inhibiting the main transcription factor that regulates iron metabolism,ironresponsive element-binding protein 2 (also known as iron regulatory protein 2),can significantly upregulate the expression of genes associated with iron metabolism (
,FTH1 and FTL) and inhibit erastin-induced ferroptosis[24].Blocking iron transport by knocking out the ferroportin gene SLC11A3 exacerbates erastin-induced ferroptosis in neuroblastoma cells[25].Furthermore,Yang
[26] observed that phosphorylase kinase catalytic subunit γ2 (PHKG2) positively regulates ferroptosis by modulating the free Fe
ion level,while inhibiting PHKG2 expression exhibits an iron-chelating effect.Autophagy can also regulate the cellular sensitivity to ferroptosis by affecting iron metabolism[27].Ferritin-selective autophagy (ferritinophagy) enhances cellular sensitivity to ferroptosis by controlling the level of available iron[28].NCOA4 is a selective cargo receptor that delivers ferritin to autophagosomes,where ferritin is degraded and free iron is released into the cytoplasm.Downregulating NCOA4 expression reduces the sensitivity of human fibrosarcoma cells (HT-1080) and human pancreatic cancer cells (PANC1) to ferroptosis.This process is regulated by autophagyrelated genes,ATG5 and ATG7[10].Other proteins that affect iron metabolism,such as NRF2[11],heat shock protein beta-1[29],and CDGSH iron-sulfur domain-containing protein 1 (CISD1,also referred to as mitoNEET)[30] can also affect cellular sensitivity to ferroptosis.
MECHANISM AND REGULATION OF FERROPTOSIS
Ferroptosis is mainly regulated by the following three mechanisms[7]:(1) regulation of iron metabolic pathways such as autophagy-related genes 5 and 7 (ATG5/ATG7)-nuclear receptor coactivator 4 (NCOA4) pathway[10] and p62-Kelch-like epichlorohydrin-associated protein-1 (Keap1)-nuclear factor erythroid 2-related factor 2 (NRF2)pathway[11];(2) regulation of lipid metabolic pathways such as the p53-serine acetyltransferase 1-arachidonate-15-lipoxygenase pathway[7],acyl-CoA synthase long-chain family member 4 (ACSL4)[12],lysophosphatidylcholine acyltransferase 3 (LPCAT3)[13],and 15-lipoxygenase/phosphatidylethanolamine (PE)-binding protein-1 (15-LOX/PEBP1)[14];and (3) regulation of the GSH/GPX4 pathway such as the cystine/glutamate antiporter system (System X
)[15],transsulfuration pathway[16],and mevalonate pathway[17].Dysregulation of these three regulatory pathways eventually significantly reduce GPX4 activity and increase intracellular lipid ROS levels,thereby leading to reduced cellular antioxidant capacity,additional lipid ROS accumulation,oxidative damage to the cell membrane,and ferroptosis.Ferroptosis suppressor protein 1 (FSP1) can inhibit lipid peroxidation and ferroptosis by directly eliminating lipid ROS independent of GPX4[18] (Figure 1).
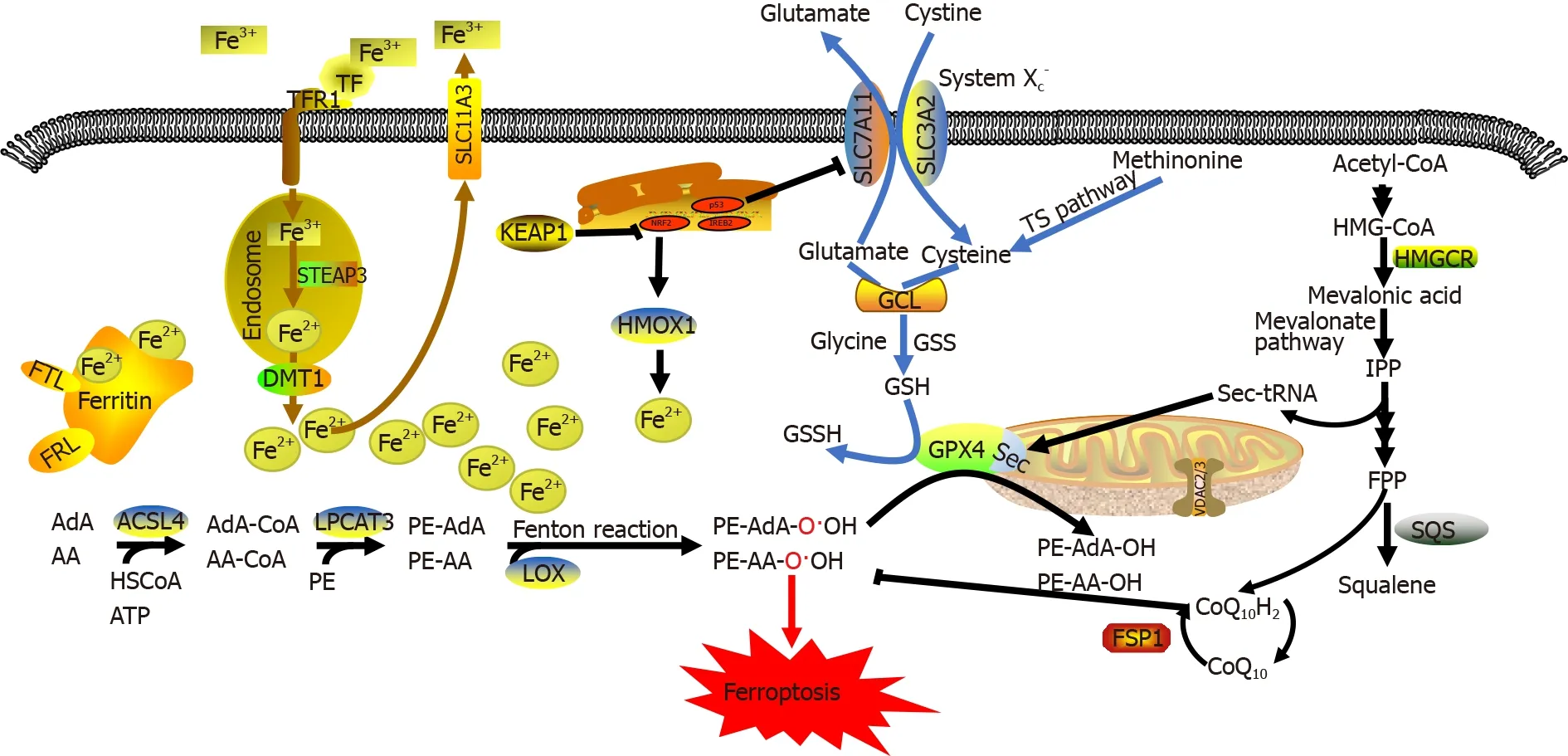
Regulation of iron metabolism
Iron is an essential trace element in the human body.Iron deficiency can cause anemia and iron-dependent enzyme abnormalities.However,iron accumulation can lead to tissue damage and increase the risk of developing various diseases (
,tumors).ROS that accumulate during cell metabolism mainly include superoxide radical anions (O
)and hydrogen peroxide (H
O
),which are converted by free Fe
ions to hydroxyl free radicals (HO
) that subsequently generate lipid peroxides by oxidizing macromolecules,especially lipid molecules (
,polyunsaturated fatty acids,PUFAs).These reactions,which involve iron and generate hydroxyl or alkoxy radicals (RO
),are termed Fenton reactions[19].Intracellular accumulation of lipid peroxides without timely elimination cause oxidative damage to DNA,proteins,and the cell membrane,eventually leading to ferroptosis[20].
Therefore,iron ions are indispensable for the accumulation of lipid peroxides and the initiation of ferroptotic pathways.The absorption,distribution,metabolism,transformation,and excretion of iron ions are closely associated with the onset of ferroptosis[21].Dietary iron is mainly absorbed as ferric (Fe
) ions in the duodenum and upper jejunum,where it is transported to the blood by transferrin.Some Fe
ions are transported by binding to the membrane receptor transferrin receptor 1 (TFR1),which are packaged into endosomes.There,Fe
ions are reduced to Fe
ions by the metalloreductase,six-transmembrane epithelial antigen of the prostate 3.Finally,Fe
ions are delivered by solute carrier family 11a2/divalent metal transporter 1 from endosomes into the cytoplasmic labile iron pool.Intracellular iron storage mainly occurs in the form of iron-protein complexes comprising ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1),while the remaining excess Fe
ions are oxidized to Fe
ions and transported out of cells by ferroportin on the cell membrane[22].
Ferroptosis,modulated by specific pathways,is involved in various biological processes and exhibits unique gene expression and molecular regulatory systems.Current studies suggest that ferroptosis is mainly caused by the imbalance between lipid ROS generation and detoxification in cells.The accumulation of lipid ROS when the cellular antioxidant capacity is reduced can result in oxidative stress-induced cell death,
ferroptosis[9].
Iron chelators can directly act on iron-containing enzymes,most likely lipoxygenases,because they can catalyze PUFA oxidation and be directly inactivated by lipophilic iron chelators.Dixon
[31] suggested that iron is extremely prone to electron exchange under aerobic conditions.Thus,the inhibition of ferroptosis by iron chelators may be attributed to the fact that iron is a cofactor of numerous important metalloenzymes and that iron chelators prevent electron transfer from iron to oxides,thereby inhibiting oxygen free radical generation and preventing ferroptosis by inhibiting lipid peroxidation.Therefore,regulating iron metabolism and ferritinophagy may serve as a new target and approach for modulating ferroptosis.
Regulation of lipid metabolism
form of CoQ10,captures free radicals that drive lipid peroxidation,thereby preventing oxidative damage to plasma membranes.FSP1 exerts its cellular protective effect against ferroptosis by catalyzing continuous CoQ10 regeneration and improving the free-radical scavenging capacity within cells.Hence,FSP1 catalyzes the synthesis of lipophilic free-radical scavengers and has a protective effect against ferroptosis caused by GPX4 deletion.It is currently believed that the FSP1/NADPH/CoQ10 pathway is independent and parallel to GPX4.Even in the absence of GPX4,FSP1,CoQ10,NADPH,and GSH serve as important antioxidants and free-radical scavengers that exhibit cellular protective effects against ferroptosis in the body.
The intracellular accumulation of lipid peroxides is the core process of ferroptosis.Lipid peroxidation in cells may be enzymatic or non-enzymatic.Non-enzymatic lipid peroxidation,also known as lipid autoxidation,is a free radical-mediated chain reaction,in which PUFAs are oxidized to lipid hydroperoxides by hydroxyl radicals generated
the Fenton reaction[19].In contrast,enzymatic lipid peroxidation refers to lipoxygenase (LOX)-catalyzed generation of various lipid hydroperoxides from free PUFAs.Then lipid hydroperoxides are catalyzed by Fe
ions to generate free alkoxy radicals,which participate in the next lipid peroxidation reaction.Continuous PUFA oxidation and depletion alters the fluid mosaic structure of cell membrane and increases its permeability,eventually leading to cell death[17,20].
In some versions of the story, the queen declares that the next queen will be the woman upon whose finger her wedding band fits. Donkeyskin, like Cinderella and her slipper59, is the only woman whose finger fits the band.
Unlike other forms of cell death,ferroptosis does not require an effector (
,poreforming proteins).Instead,lipid-mediated oxidative stress and subsequent membrane damage are key factors leading to the onset of ferroptosis.In particular,PUFAs,which contain bis-allylic protons that are vulnerable to hydrogen abstraction,are more likely to form lipid peroxides and induce ferroptosis[26].PUFA abundance and localization determine the degree of lipid peroxidation in cells,and thus,the extent of ferroptosis[7].
Fe
ions participate in the formation of free radicals and are an important catalyst in lipid peroxidation.Free PUFAs serve as substrates for the synthesis of lipid signaling mediators,but they must be esterified to membrane phospholipids and oxidized to become ferroptotic signals[17].These toxic mediators are sparsely distributed within the cell membrane,mitochondrial membranes,lysosomal membranes,and endoplasmic reticulum membranes[26].A lipidomic study uncovered that lipid metabolism disorders are closely associated with ferroptosis,where the key phospholipids—PEs—which contain arachidonic acid (C20:4) or its derivative adrenic acid (C22:4),are oxidized to ox-phosphatidylethanolamines (ox-PEs) that induce the onset of ferroptosis[34].PUFAs are converted to coenzyme A derivatives,which are incorporated into phospholipids to become ferroptotic signals.Thus,the regulatory enzymes involved in PUFA biosynthesis from membrane phospholipids can trigger or prevent ferroptosis.Indeed,PUFA formation requires various lipid metabolism enzymes,such as ACSL4[14],LPCAT3[13],and 15-LOX/PEBP1[14].In addition,lipid peroxidation promotes ferroptosis due to the generation of toxic aldehydes,such as 4-hydroxy-2-nonenal and malondialdehyde that can inactivate some proteins involved in normal physiological functions[35].
In addition to the above-mentioned metabolic regulatory pathways,other cellular pathways are involved in the regulation of ferroptosis.Bersuker
[18] and Doll
[53] found that the FSP1/coenzyme Q (CoQ)/NADPH pathway also inhibits ferroptosis.FSP1 was previously known as apoptosis-inducing-factor mitochondriaas-2.Both research groups found that FSP1 exhibited CoQ oxidoreductase activity,which mediates NAD(P)H-dependent CoQ10 regeneration.Ubiquinol,the reduced
ACSL4,which belongs to the long-chain acyl-CoA synthetase family,catalyzes the activation of fatty acids to form fatty acyl CoA in the body.It is also the key enzyme required in the first step of fatty acid catabolism.Previous studies have revealed that knocking out enzymes of the ACSL family other than ACSL4 in mouse embryonic fibroblasts does not cause ferroptosis[12].Unlike other members of the ACSL family,ACSL4 can activate long-chain PUFAs and participate in the synthesis of membrane phospholipids.For example,ACSL4 catalyzes the conversion of arachidonic acid and adrenergic acid to arachidonoyl-CoA and adrenyl-CoA,respectively,which participate in the synthesis of negatively charged membrane phospholipids (
,phosphatidylethanolamines and phosphatidylinositol) and their incorporation into the cell membrane.LPCAT3 knockout cells display only a slight alleviation of ferroptosis compared to ACSL4 knockout cells.Additionally,ACSL4 is required for lipid peroxides to inhibit GPX4[12,36].These results suggest that ACSL4 may be a crucial determinant of ferroptosis.Another study revealed that thiazolidinediones exhibit a protective effect on ACSL4-knockout embryonic fibroblasts.The combination of thiazolidinediones and RSL3 alleviated membrane lipid oxidation and cell death and significantly improved the survival of ACSL4-knockout mice[12].Hence,ACSL4 inhibition may be a new target for the treatment of diseases associated with ferroptosis.
LOXs are non-heme,iron-containing enzymatic effector proteins essential for mediating the formation of ferroptosis-related peroxides.Knocking out LOXs,which prefer free PUFAs as substrates,can alleviate erastin-induced ferroptosis and cellular damage[3].Vitamin E can inhibit LOX activity,which provides a foundation for the protective effect of vitamin E against ferroptosis[37].Current studies suggest that LOXs primarily form a complex with PEBP1,which allosterically regulate LOXs to accommodate the ferroptotic signal sn2-15-Hydroperoxy-eicasotetraenoyl-phosphatidylethanolamines (sn2-15-HpETE-PE) at the catalytic site.Two major LOX subtypes mediate lipid peroxidation:15-LO1 and 15-LO2.These two LOX subtypes have tissuespecific distribution patterns.For example,15-LO1 is highly expressed in human aortic endothelial cells,while 15-LO2 is highly expressed in renal tubular endothelial cells and neuronal cells[14].A previous redox metabolomic analysis revealed the similarity between 15-LO1 and 15-LO2.Both enzymes are involved in ferroptosis-associated diseases,such as traumatic brain injury,asthma,and acute renal ischemic injury[7].LOX-mediated free PUFA oxidation requires 15-LOX/PEBP1 complex formation.In this complex,PEBP1 allosterically regulates LOXs and initiates downstream phospholipase A
-related oxidation pathways for specific PUFAs.PEBP1,also known as RAF1 kinase inhibitor protein,is a small scaffold protein that binds to RAF1 and inhibits activity under steady-state conditions.15-LOs are newly identified partners of PEBP1.15-LO/PEBP1 complexes allosterically activate LOXs,which convert 15-hydroxyperoxyeicosatetraenoic acid (15-HpETE) to the pro-ferroptotic signal,15-HpETE-PE,thereby triggering ferroptosis[38].The mechanism by which the LOX/PEBP1 complex selects specific PUFAs for oxidation among diverse unsaturated fatty acids remains unknown.Clearly,this issue urgently needs to be addressed in investigating the regulatory mechanism of ferroptosis.
Regulation of amino acid metabolism
Amino acid metabolism is an important component of metabolic networks,and amino acid metabolism disorders are closely associated with ferroptosis[7].GSH is an important antioxidant and free radical scavenger in the body.Many free radicals produced
metabolism can damage cell membranes,attack biological macromolecules,promote aging,and induce the onset of tumors or atherosclerosis.Functionally,GSH can bind and convert harmful,toxic molecules (
,free radicals and heavy metals) into harmless substances that can be excreted from the body[39].GSH is a tripeptide consisting of three amino acid residues:glutamic acid,cysteine,and glycine.It exists in reduced (G-SH) and oxidized (G-S-S-G) forms and is the first line of defense for free-radical scavenging in the body due to the presence of an active sulfhydryl (-SH) group that is susceptible to oxidization and dehydrogenation.Together with non-enzymatic antioxidants (reduced nicotinamide adenine dinucleotide phosphate/nicotinamide adenine dinucleoside phosphate),GSH exerts a strong protective effect on the body[40].The synthesis of GSH requires cysteine as the starting material.Therefore,cellular resistance to lipid oxidation relies on intracellular cysteine levels,which are mainly produced by the System X
and transsulfuration pathways.
System X
plays an important role in maintaining GSH homeostasis and distribution.This molecule is a disulfide-linked heterodimer that comprises the regulatory subunit solute carrier family 3 member 2 (SLC3A2) and the catalytic subunit solute carrier family 7 member 11 (SLC7A11).System X
promotes a 1:1 cystine and glutamic acid exchange across the plasma membrane.Cystine is reduced to cysteine upon entering cells[41].Thus,System X
regulates GSH synthesis by affecting extracellular glutamic acid levels[42].A previous study found that System X
-knockout mice have significantly lower glutamic acid levels around neurons and a milder drug-induced neurotoxic response than normal mice[43].Previous pharmacological studies revealed that erastin,sulfasalazine,and high glutamic acid concentrations induce ferroptosis by inhibiting System X
[3,44].These findings indicate that System X
may mediate ferroptosis initiation by affecting glutamic acid uptake and GSH synthesis.
Methionine can be converted to adenosylhomocysteine and cysteine in cells
the transsulfuration pathway[16].During cysteine insufficiency,homocysteine is converted to cystathionine (a cysteine precursor),which eventually enters the cysteine pool
the transsulfuration pathway.Numerous studies have demonstrated that more than 40% of cysteine in mammals is obtained from food.Cysteine is mainly used to synthesize GSH,antioxidant peptides,and thioredoxin (Trx) in the body.Under oxidative stress,cystathionine-b-synthetase promotes the conversion of methionine to cysteine and subsequent GSH synthesis,thereby protecting cells from oxidative stressinduced damage[16,45].Hence,cysteine can be synthesized in cells
the transsulfuration pathway even when intracellular System X
is inhibited,indicating that ferroptosis inducers,which inhibit System X
,cannot completely and effectively kill cells.Hayano
[46] showed that inhibiting cysteinyl-tRNA synthetase (CARS)expression using RNA interference upregulates the transsulfuration pathway and enhances cellular resistance to erastin-induced ferroptosis but is unable to inhibit RSL3-or buthionine sulfoximine-induced ferroptosis,suggesting that the transsulfuration pathway negatively regulates ferroptosis.
Glutamic acid and glutamine are additional ferroptosis regulators.A high extracellular concentration of glutamic acid can inhibit System X
and trigger ferroptosis.Ottestad-Hansen
[43] found that knocking out System X
protects mice against neurotoxic injuries caused by glutamic acid accumulation.Additionally,iron chelators and ferroptosis inhibitors can inhibit glutamic acid-mediated neurotoxicity.Glutamine naturally exists in human tissues and plasma at substantial concentrations.Its degradation fuels the tricarboxylic acid cycle and provides fundamental materials for biosynthetic processes.During glutamine deficiency or the inhibition of glutamine degradation,ROS accumulation,lipid peroxidation,and ferroptosis cannot be induced by depleting cysteine or blocking cystine uptake,probably because the product of glutamine degradation,α-ketoglutarate,is essential for the onset of ferroptosis[3].However,not all glutamic acid metabolic pathways can induce ferroptosis.The first step of glutamic acid metabolism is the conversion of glutamine into glutamic acid by the glutaminases GLS1 and GLS2.These glutaminases have similar structures and enzymatic properties,but only GLS2 can induce ferroptosis,probably because GLS2 is a transcriptional target of p53.Indeed,GLS2 upregulation can induce p53-dependent ferroptosis[47,48].Under certain circumstances,p53 can suppress ferroptosis by blocking dipeptidyl-peptidase-4 activity in a transcription-independent manner[49].Inhibiting glutamine degradation has been demonstrated to alleviate cardiac,renal,and brain injury caused by ischemia-reperfusion in an experimental model[50].Hence,the regulation of glutamine anabolism may provide new approaches for alleviating ferroptosis-induced organ injuries.
In addition,oxidant/antioxidant imbalance may also induce ferroptosis[7].ROS levels in the body are regulated by an antioxidative defense system comprising antioxidants,such as Nrf2,GPX4,and catalase.However,inhibitors of the antioxidative system (
,superoxide dismutase inhibitors and thioredoxin reductase inhibitors) can induce human epithelial/fibroblast cell death only when intracellular GSH is depleted[51],indicating that erastin may induce ferroptosis by interacting with a specific downstream target of GSH.GPX4,which belongs to the GPX antioxidative defense system,is a key enzyme in maintaining the balance between GSH and GS-SG.High SLC7A11 expression in various tumor types increases cystine uptake and GPX4 synthesis in cells,thereby promoting tumor growth by reducing cellular oxidative stress and inhibiting ferroptosis[41].
The King had a walnut shell ready, and the trial began; but not one of the dogs the two eldest23 sons had brought with them would in the least fit into the shell
Voltage-dependent anion channels (VDACs) are transmembrane channels located on the outer mitochondrial membrane that transport ions and metabolites.VDACs regulate mitochondrial metabolism and energy production and participate in regulating signaling pathways,leading to both cell survival and death.There are numerous VDAC subtypes including VDAC1,VDAC2,and VDAC3.The open state of VDACs mediates the influx of respiratory substrates,ADP,and phosphoric acid into the mitochondria,while its closure blocks transport across mitochondrial membranes[54].Tubulin,a globular protein on VDACs,can dynamically regulate mitochondrial metabolism and ion transportation by blocking VDACs[55].Tubulin-induced VDAC closure restricts metabolite influx into the mitochondria and limits ATP production,leading to attenuated oxidative stress due to the inhibition of mitochondrial metabolism and a relatively low ATP/ADP ratio.Erastin can inhibit the effect of tubulin on VDACs and maintain an open state by preventing free tubulins in the cytoplasm from blocking VDACs.The open VDAC state leads to increased mitochondrial metabolism,decreased glycolysis,and elevated ROS production.Exposure of VDACs to the ferroptosis inducer,erastin,causes increased permeability of outer mitochondrial membranes,membrane ion channel opening,and disrupted cellular homeostasis,which results in dysfunctional mitochondrial metabolism and oxidation,increased ROS production,and enhanced lipid peroxidation,eventually triggering ferroptosis[56].A previous study showed that inhibiting VDAC2 or VDAC3 expression renders cells insensitive to erastin-induced ferroptosis,but upregulating VDAC2 or VDAC3 expression does not significantly increase cellular sensitivity to erastin-induced ferroptosis.These data suggest that despite being involved in the regulation of ferroptosis,neither VDAC2 nor VDAC3 is a prerequisite of ferroptosis[57].VDAC1 is closely related to the onset of ferroptosis,as it mainly maintains calcium homeostasis and ROS levels in the mitochondria.
GPX4 is a GSH-dependent enzyme.Selenocysteine is an amino acid within the catalytic center of GPX4,but since it is encoded by a UGA codon (which is also a stop codon),selenocysteine needs to be inserted into GPX4 by a specific carrier.Selenocysteine-specific tRNA (sec-tRNA) contains isopentenyladenosine and can decode the selenocysteine UGA codon,thereby allowing the accurate insertion of selenocysteine into corresponding proteins.Importantly,sec-tRNA maturation can also be regulated by the mevalonate pathway acting on GPX4 because its maturation requires tRNAisopentenyltransferase to catalyze the transfer of the isopentenyl group of isopentenyl pyrophosphate (IPP) to the specific adenine sites of sec-tRNA precursors.Since IPP is an important product of the mevalonate pathway,inhibitors of the mevalonate pathway (
,statins) can inhibit sec-tRNA maturation and GPX4 synthesis[16,17],thereby affecting the progression of ferroptosis.IPP and mevalonate pathway inhibitors regulate the onset of ferroptosis by affecting GPX4.At present,GPX4 is a key target to induce ferroptosis and is activated by numerous ferroptosis inducers,such as erastin and RSL3.Erastin inhibits GPX4 activity by depleting GSH,while RSL3 directly inhibits GPX4 activity[7],resulting in lipid peroxide accumulation that triggers ferroptosis.Additionally,other ferroptosis inducers (
,diphenylene iodonium (DPI),DPI7,DPI10,and DPI12) exert similar effects by directly inhibiting GPX4 activity.Knocking out GPX4 Leads to excess intracellular lipid peroxide accumulation and cell death[52].Therefore,GPX4 is an important target for triggering ferroptosis.
OTHER REGULATORY PATHWAYS
FSP1/CoQ/NADPH pathway
PUFAs are activated by ACSL4 and transported by LPCAT3 to the inner and outer leaflets of the cell membrane,where they undergo esterification and participate in the oxidation of negatively charged membrane phospholipids.Under normal circumstances,15-LOX/PEBP1 and GPX4 co-regulate the oxidation of esterified fatty acids,but during oxidant/antioxidant imbalance,long-chain PUFAs in the cell membrane are often oxidized and trigger ferroptosis,especially when being induced by other factors such as RSL3[22].
Lipids are important regulators of cell death.In mammals,both apoptotic and nonapoptotic pathways can be induced,regulated,or inhibited by different lipid signals[34].For example,increasing the intracellular saturated fatty acid-to-monounsaturated fatty acid ratio can trigger apoptotic pathways.Increased long-chain fatty acid levels can trigger necrotic pathways[32],and exogenous monosaturated fatty acids can reduce cell death
acyl-CoA synthetase long chain family member 3 (ACSL3).All of these pathways exert a lipotoxic effect[33].
Voltage-dependent anion channels
A grave! dig me agrave! sounded again in her ears, and she would have gladly buriedherself, if in the grave she could have found forgetfulness of heractions
FERROPTOSIS INDUCERS AND INHIBITORS
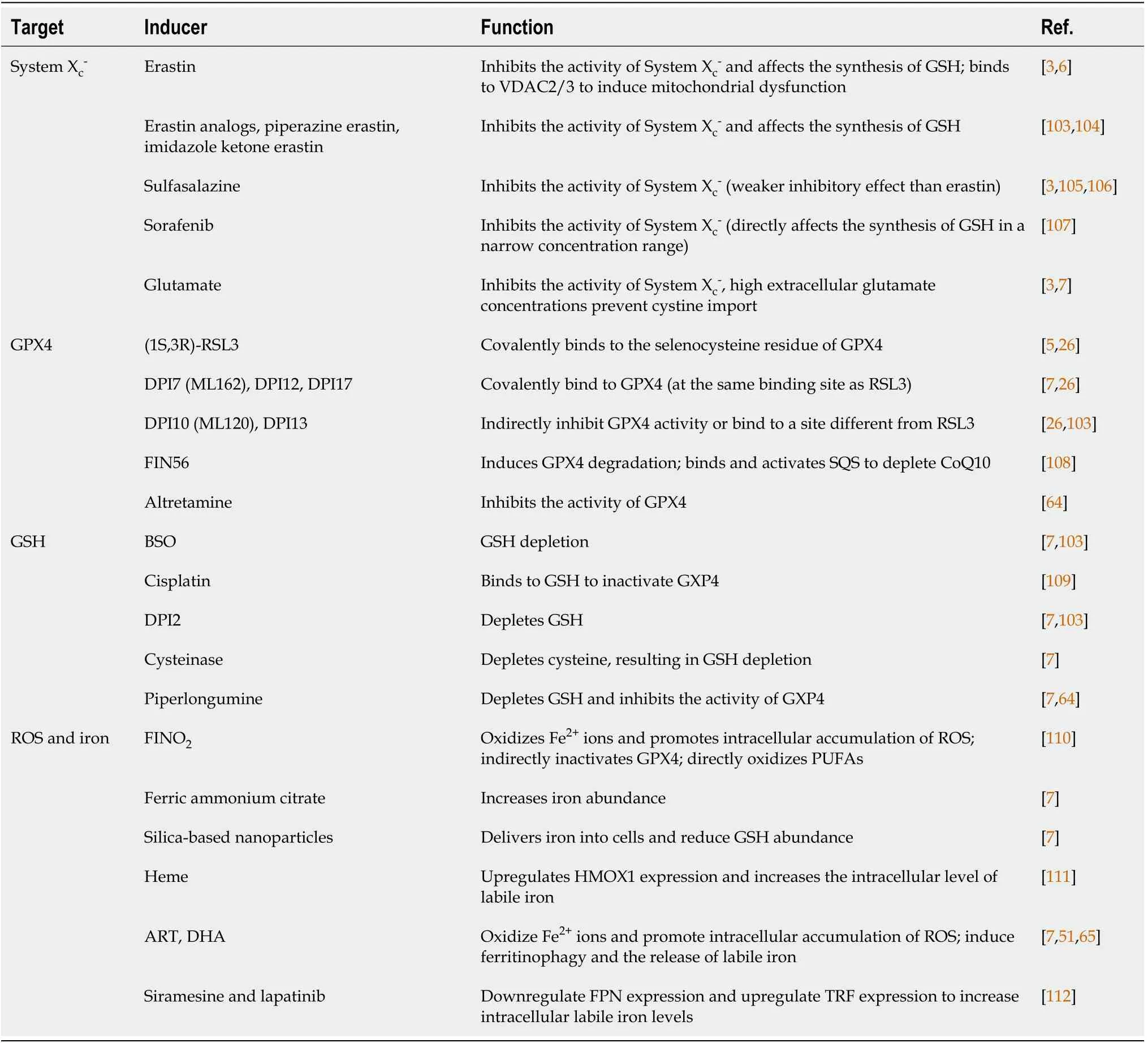
Common ferroptosis inducers
Ferroptosis inducers can be divided into four categories according to their targets(Table 1):System X
;GPX4;GSH;and iron ions and ROS.
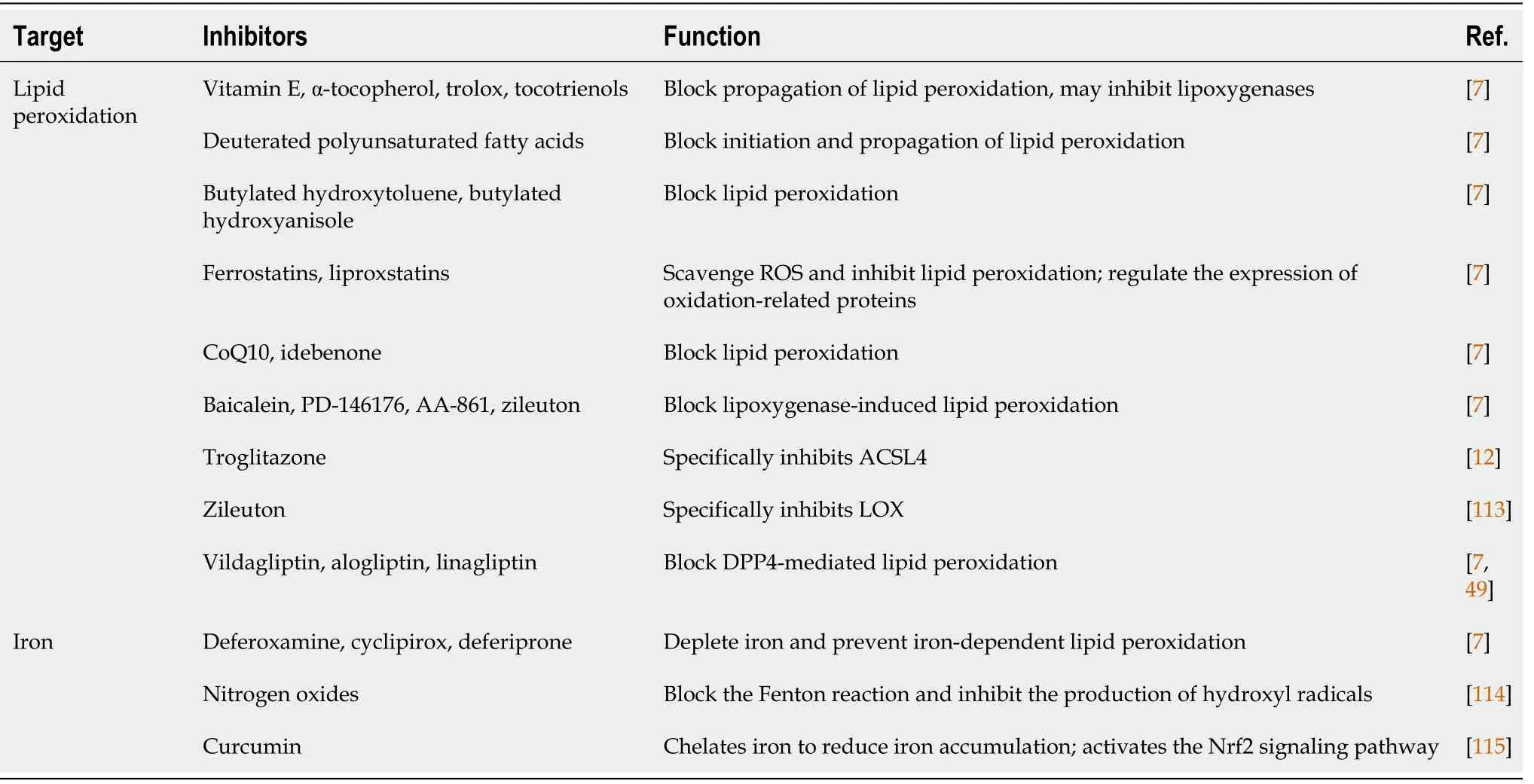
Common ferroptosis inhibitors
Ferroptosis inhibitors can be divided into two categories according to their mechanisms of action (Table 2):reduction of intracellular iron accumulation;and inhibition of lipid peroxidation.
FERROPTOSIS AND GI DISEASES
Numerous studies have demonstrated that ferroptosis leads to cell death in GI tumors(
,pancreatic,liver,colorectal,and gastric cancers) and plays an important role in inhibiting tumor growth.Therefore,inducing ferroptosis in tumor cells is expected to become a novel therapeutic strategy.Although only limited
and
experiments on ferroptosis inducers have been conducted,a few small-molecule ferroptosis inducers have been discovered that display excellent therapeutic or synergistic outcomes against tumors.
Ferroptosis and pancreatic cancer
Pancreatic cancer is a highly malignant GI tumor with a poor prognosis.Although there are drugs available to treat pancreatic cancer,patients receiving pharmacotherapy rarely survive more than 6 mo.Gemcitabine is the first-line chemotherapeutic agent for pancreatic cancer,but pharmacotherapy and immunotherapy still fail to yield an ideal therapeutic outcome.Therefore,it is imperative to develop new strategies for enhancing the sensitivity of pancreatic cancer to immunotherapy and reducing its resistance to gemcitabine[58].Tang
[59] utilized public databases to systematically analyze the expression of 43 ferroptosis regulators in 31 cancer types and constructed a highly accurate prognostic prediction model for pancreatic cancer based on ferroptosis regulators.A follow-up investigation on the effect of ferroptosis on the tumor microenvironment revealed that tumors that are highly sensitive to ferroptosis may also be sensitive to immune checkpoint inhibitors and
.The authors also found that gemcitabine-resistant cancer cells had increased expression levels of SLC7A11 and SLC3A2,but their effects on ferroptosis sensitivity require further investigation.Zhu
[60] found that heat shock protein family A55 (HSPA5)is closely associated with the prognosis of pancreatic cancer patients who received gemcitabine treatment.Activating the HSPA5-GPX4 pathway in pancreatic cancer cells may lead to gemcitabine resistance that may be reversed by inhibiting HSPA5 or GPX4 expression,which may also induce ferroptosis.Shintoku
[61] demonstrated that erastin and RSL3 can induce pancreatic cancer cell death,and LOXs can increase the sensitivity of tumor cells with mutated RAS to erastin and RSL3.A subsequent study by Kuang
[62] showed that the redox regulator quinazolindione (QD) inhibits pancreatic cancer cell proliferation by inducing ferroptosis.Further,the compound QD325 significantly inhibits the growth of transplanted tumors in mice and is well tolerated
.Kasukabe
[63] showed that the combination of cotylenin A (CNA) and phenethyl isothiocyanate significantly inhibits pancreatic cancer cell proliferation by promoting ferroptosis.A study carried out by Yamaguchi
[64]suggested that piperlongumine could synergistically kill human pancreatic cancer cells with CN-A or sulfasalazine
ferroptosis.In recent years,some Chinese herbal medicines have also been found to exert antitumor effects by inducing ferroptosis.Previous
and
assays showed that the antimalarial drug,artesunate,could cause excessive intracellular ROS accumulation by promoting lipid peroxidation and regulating iron metabolism.Additionally,artesunate can specifically induce ferroptosis in pancreatic cancer cells with a mutated
gene while exerting minimal toxic effects on normal cells[65],primarily by increasing ROS production[51].A further study revealed that inhibiting glucose regulatory protein 78 expression reverses the resistance of pancreatic cancer cells to ferroptosis and enhances the sensitivity of tumors to artesunate[66].The animal model constructed by Badgley
[67] showed that therapeutic cysteine depletion can induce ferroptosis in pancreatic tumors in mice with mutated
/
.However,Dai
[68] recently found that ferroptosis can promote dead cancer cells to release KRAS protein,which will then be packaged into exosomes and taken up by macrophages.Then,the macrophages undergo polarization to M2 macrophages,which promote the malignant growth of pancreatic cancer.These results indicate that ferroptosis may exhibit complicated biological effects in the treatment of pancreatic cancer.
Horror gripped the heart of the World War I soldier as he saw his lifelong friend fall in battle. Caught in a trench1 with continuous gunfire whizzing over his head, the soldier asked his lieutenant2 if he might go out into the No Man s Land between the trenches3 to bring his fallen comrade back.
Ferroptosis and liver cancer
Surgery is the most important therapeutic approach for patients with hepatocellular carcinoma (HCC),but the rate of postoperative recurrence and metastasis is relatively high.Sorafenib is a commonly used chemotherapeutic drug against HCC,but it is difficult to clinically determine the prognosis of HCC and reduce sorafenib resistance[69].Shan
[70] analyzed two different public HCC databases and found that ubiquitin-like modifier activating enzyme 1 (UBA1) can regulate ferroptosis in HCC cells
the Nrf2 pathway.The authors subsequently confirmed that silencing UBA1 gene expression inhibits HCC proliferation,migration,and invasion,increasing Fe
and MDA levels in cancer cells.These results indicate that UBA1 can be used as an independent indicator of liver cancer progression.Liang
[71] systematically analyzed the expression of 60 ferroptosis-associated genes in HCC tumor tissues and their relationships with the overall survival of patients.The authors proposed and validated a prognostic model comprising 10 ferroptosis-associated genes (ACACA,ACSL3,CISD1,CARS,G6PD,GPX4,NQO1,NFS1,SLC7A11,and SLC1A5).These efforts provided an important approach for elucidating mechanisms underlying HCC development and predicting its prognosis.
Of course, Missy. Of course. I opened the door and slowly followed Brother Lu back to the living room. Mom and Dad still hadn t come in yet. I figured they were sitting in the car, preparing Dad for what to do or say when he saw me. Mom knew how afraid I was. But it wasn t fear that my father would yell5 at me or be angry with me. I wasn t afraid of him. It was the sadness in his eyes that frightened me. The knowledge that I had been in trouble and pain, and had not come to him for help and support. The realization6 that I was no longer his little girl.
Studies on the mechanisms through which sorafenib and erastin induce ferroptosis in HCC have provided new approaches for addressing chemotherapeutic drug resistance.Louandre
[72] showed that sorafenib-treated HCC cells had significantly lower retinoblastoma protein expression than untreated HCC cells,with a mortality rate two to three times higher than that of the untreated group.Subsequently,
experiments on mice implanted with HCC cells and
experiments on shRb-transfected Huh7 cells clarified the mechanism through which Rb regulates sorafenib-induced ferroptosis in HCC.Sorafenib induces ferroptosis in HCC by enhancing mitochondrial ROS generation,while Rb inactivation aggravates ferroptosis by increasing mitochondrial ROS levels and oxidative stress.Sun
[11]reported that the p62-Keap1-Nrf2 signaling pathway plays an important role in erastin/sorafenib-induced ferroptosis in HCC,where it modulates ferroptosis by regulating the expression of downstream iron-and ROS metabolism-related genes.Interfering with p62 expression can enhance erastin/sorafenib-induced ferroptosis in HCC.Additionally,experiments in Nrf2-shRNA-transfected HCC cells and mice implanted with Nrf2-shRNA-transfected HCC cells showed that Nrf2 knockdown enhances the antitumor activity of erastin/sorafenib against HCC[73].Qi
[74]found that erastin significantly inhibits the expression of GA binding protein transcription factor subunit β 1 (GABPB1) protein and peroxidase genes in HCC cells,thereby resulting in intracellular ROS and malondialdehyde accumulation,which leads to cell death.Therefore,GABPB1 may be a key molecule that mediates erastininduced ferroptosis in HCC.Further,ACSL3 and ACSL4 expression is significantly upregulated in HCC[75],and ACSL4 contributes to erastin-induced ferroptosis
5-hydroxyeicosatetraenoic acid-mediated lipotoxicity[14].Additional studies[76-78]have shown that inhibiting metallothionein 1G and oxidative stress-related protein sigma 1 receptor enhances the sensitivity of liver cancer cells to sorafenib by inducing ferroptosis.Wang
[79] identified and explored branched-chain amino acid aminotransferase 2 (BCAT2),which is involved in System X
inhibitor-induced ferroptosis in liver cancer.In addition,BCAT2 also participates in ferroptosis synergistically induced by sulfasalazine and sorafenib.
Combination therapy may improve the clinical outcomes of patients with liver cancer by partially addressing the issue of drug resistance.Low-density lipoprotein nanoparticles reconstituted with the natural omega-3 PUFA,docosahexaenoic acid(LDL-DHA),can effectively kill liver cancer cells by triggering ferroptosis[80].The combined treatment of liver cancer cells with erastin,sorafenib,and haloperidol can elevate intracellular iron ion concentrations,which generate excessive ROS
the Fenton reaction and increase lipid oxidation,thereby inducing ferroptosis in liver cancer cells[77].Shang
[81] found that ceruloplasmin (CP) inhibits ferroptosis by regulating iron homeostasis in HCC cells,while inhibiting CP significantly increases intracellular Fe
and ROS accumulation,thereby promoting erastin-and RSL3-induced ferroptosis in HCC.Li
[82] reported that sorafenib and artesunate synergistically suppress liver cancer by inducing ferroptosis.Further,nanoparticlebased drugs also offer a new direction for
induction of ferroptosis in liver cancer.Tang
[83] showed that manganese-silica nanodrugs induce ferroptosis in tumor cells by rapidly depleting intracellular GSH.LDL-DHA nanoparticles increase lipid peroxidation in liver cancer cells,reduce GSH levels,and inhibit GPX4 activity,thereby inducing ferroptosis that kills liver cancer cells and inhibits the
growth of liver tumors in rats[80].
Foolish, foolish child! said the mother pig, looking quite distressed10. And you, Blacky? turning to her youngest son, what sort of a house shall I order for you?
Ferroptosis and gastric cancer
Gastric cancer (GC) is among the most common causes of cancer-related deaths worldwide,with nearly one million cases diagnosed each year and more than 730000 deaths.Conventional treatments for GC include surgery,chemotherapy,and radiotherapy.Chemotherapy,despite being the primary therapeutic approach,causes significant side effects for most patients and often cannot cure patients with advanced GC[84].Therefore,it is necessary to develop a better therapeutic approach for GC.Lee
[85] found that the sensitivity of GC cells to ferroptosis depends on PUFA biosynthesis.Stearoyl-CoA desaturase 1 (SCD1) promotes tumor growth and makes GC cells resistant to ferroptosis.Notably,GC patients with high SCD1 expression may not have an optimistic prognosis.Taken together,this study provides new insights into the potential of SCD1 as a biomarker and therapeutic target for GC[86].Hao
[87]found that inhibiting cysteine dioxygenase 1 (CDO1) expression could inhibit ferroptosis in GC by upregulating GPX4 expression and preventing ROS production.Sun
[88] showed that perilipin2 inhibits ferroptosis in GC by regulating ACSL3 and 15-LOX.Some ingredients of Chinese medicines,such as
planch[89] and Tanshinone IIA[90] also exhibit anticancer effects against GC by participating in ferroptosis.
When the festivities ended, Thelma quickly rose from her seat and rushed over to the man. Pardon me, Thelma said. Please forgive me if I made you feel uncomfortable by staring at you all night. I just couldn t help myself from looking your way. You see, you look just like my fifth husband.
When the little man arrived on the following day she began with Kasper, Melchior, Belshazzar,33 and all the other names she knew, in a string, but at each one the manikin called out: That s not my name
Ferroptosis and colorectal cancer
Colorectal cancer (CRC) is the most common malignant GI tumor that poses a major threat to human health.Recently,an increasing trend in CRC incidence and fatality rates has been observed,resulting from improved living standards and dietary changes[91,92].A previous study showed that the ferroptosis inducer RSL3 triggers ferroptosis in various CRC cell types by affecting GPX4 activity in a dose-and timedependent manner[93].Acyl-CoA dehydrogenase,short/branched chain (ACADSB),which belongs to the acyl-CoA dehydrogenase family,reduces GSH concentration by negatively regulating GSH reductase and GPX4 expression.Further,ACADSB affects CRC cell migration,invasion,and proliferation by regulating ferroptosis[94].Another study on ferroptosis-related mechanisms in CRC laid the foundation for the development of anticancer drugs against CRC.Park
[95] showed that bromelain affects ferroptosis by regulating ACSL4 expression in CRC cells with Kras mutations.Additionally,talaroconvolutin A[96],2-imino-6-methoxy-2H-chromene-3-carbothioamide[97],and resibufogenin[98] have been found to inhibit CRC cell proliferation and tumorigenesis by modulating ferroptosis in CRC cells.Some studies also found that combination therapy could partially address the issue of CRC resistance to chemotherapeutic drugs
ferroptosis.Andrographis enhances the sensitivity of CRC cells to 5-fluorouracil by promoting ferroptosis[99].The combination therapy using the natural products β-elemene and cetuximab can kill CRC cells with mutated KRAS genes by inducing ferroptosis and inhibiting epithelial-mesenchymal transition[100].The combination of high-dose vitamin C and cetuximab can improve the drug sensitivity of CRC by triggering ferroptosis,thereby laying the foundation for the treatment of CRC[101].
CONCLUSION
Ferroptosis has received increasing attention since being proposed as a form of RCD by Dixon
[3] in 2012.Numerous in-depth studies have been conducted on the complex molecular mechanisms underlying ferroptosis.These studies facilitate a deeper understanding of the onset and progression of ferroptosis-associated diseases.The further development of relevant targeted drugs has also led to the emergence of a new research field associated with ferroptosis onset and progression for the treatment of GI tumors[59].Following the discovery of erastin in 2003,numerous ferroptosis inducers and inhibitors have been identified because of the increasing importance of the relationship between ferroptosis and GI tumors[7].Sorafenib,the sole first-line drug for liver cancer,is believed to kill hepatocytes
ferroptosis.Additionally,some
and
drug trials on pancreatic cancer have provided new theoretical bases and research directions for the pharmacotherapy of pancreatic cancer.Some studies on ferroptosis in GC and CRC indicated that inducing ferroptosis could cause cell death in GI tumors and exert a synergistic effect with other anticancer drugs,thereby enhancing tumor sensitivity to existing treatments.Hence,inducing ferroptosis may have considerable potential for treating GI tumors[102].However,research on ferroptosis is still at a preliminary stage,and it is of great theoretical and practical significance to continuously explore the mechanisms and roles of ferroptosis in various diseases.These studies will reveal highly effective and targeted therapeutic approaches.For instance,the mechanism and key regulators of ferroptosis as well as its relationships with tumor-associated genes and other RCDs (
,autophagy and apoptosis),are potential directions and goals for future studies.Collectively,these studies will facilitate an in-depth understanding of the molecular mechanism through which GI tumors evade cell death and promote the development of novel effective therapeutic strategies.Therefore,further discoveries and investigation of ferroptosis inducers and inhibitors will provide a theoretical foundation and new method for the treatment of GI tumors.
1 Galluzzi L,Vitale I,Aaronson SA,Abrams JM,Adam D,Agostinis P,Alnemri ES,Altucci L,Amelio I,Andrews DW,Annicchiarico-Petruzzelli M,Antonov AV,Arama E,Baehrecke EH,Barlev NA,Bazan NG,Bernassola F,Bertrand MJM,Bianchi K,Blagosklonny MV,Blomgren K,Borner C,Boya P,Brenner C,Campanella M,Candi E,Carmona-Gutierrez D,Cecconi F,Chan FK,Chandel NS,Cheng EH,Chipuk JE,Cidlowski JA,Ciechanover A,Cohen GM,Conrad M,Cubillos-Ruiz JR,Czabotar PE,D'Angiolella V,Dawson TM,Dawson VL,De Laurenzi V,De Maria R,Debatin KM,DeBerardinis RJ,Deshmukh M,Di Daniele N,Di Virgilio F,Dixit VM,Dixon SJ,Duckett CS,Dynlacht BD,El-Deiry WS,Elrod JW,Fimia GM,Fulda S,García-Sáez AJ,Garg AD,Garrido C,Gavathiotis E,Golstein P,Gottlieb E,Green DR,Greene LA,Gronemeyer H,Gross A,Hajnoczky G,Hardwick JM,Harris IS,Hengartner MO,Hetz C,Ichijo H,J??ttel? M,Joseph B,Jost PJ,Juin PP,Kaiser WJ,Karin M,Kaufmann T,Kepp O,Kimchi A,Kitsis RN,Klionsky DJ,Knight RA,Kumar S,Lee SW,Lemasters JJ,Levine B,Linkermann A,Lipton SA,Lockshin RA,López-Otín C,Lowe SW,Luedde T,Lugli E,MacFarlane M,Madeo F,Malewicz M,Malorni W,Manic G,Marine JC,Martin SJ,Martinou JC,Medema JP,Mehlen P,Meier P,Melino S,Miao EA,Molkentin JD,Moll UM,Mu?oz-Pinedo C,Nagata S,Nu?ez G,Oberst A,Oren M,Overholtzer M,Pagano M,Panaretakis T,Pasparakis M,Penninger JM,Pereira DM,Pervaiz S,Peter ME,Piacentini M,Pinton P,Prehn JHM,Puthalakath H,Rabinovich GA,Rehm M,Rizzuto R,Rodrigues CMP,Rubinsztein DC,Rudel T,Ryan KM,Sayan E,Scorrano L,Shao F,Shi Y,Silke J,Simon HU,Sistigu A,Stockwell BR,Strasser A,Szabadkai G,Tait SWG,Tang D,Tavernarakis N,Thorburn A,Tsujimoto Y,Turk B,Vanden Berghe T,Vandenabeele P,Vander Heiden MG,Villunger A,Virgin HW,Vousden KH,Vucic D,Wagner EF,Walczak H,Wallach D,Wang Y,Wells JA,Wood W,Yuan J,Zakeri Z,Zhivotovsky B,Zitvogel L,Melino G,Kroemer G.Molecular mechanisms of cell death:recommendations of the Nomenclature Committee on Cell Death 2018.
2018;25:486-541 [PMID:29362479 DOI:10.1038/s41418-017-0012-4]
2 Zheng H,Jiang J,Xu S,Liu W,Xie Q,Cai X,Zhang J,Liu S,Li R.Nanoparticle-induced ferroptosis:detection methods,mechanisms and applications.
2021;13:2266-2285[PMID:33480938 DOI:10.1039/d0nr08478f]
3 Dixon SJ,Lemberg KM,Lamprecht MR,Skouta R,Zaitsev EM,Gleason CE,Patel DN,Bauer AJ,Cantley AM,Yang WS,Morrison B 3rd,Stockwell BR.Ferroptosis:an iron-dependent form of nonapoptotic cell death.
2012;149:1060-1072 [PMID:22632970 DOI:10.1016/j.cell.2012.03.042]
4 Dolma S,Lessnick SL,Hahn WC,Stockwell BR.Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells.
2003;3:285-296 [PMID:12676586 DOI:10.1016/s1535-6108(03)00050-3]
5 Yang WS,Stockwell BR.Synthetic lethal screening identifies compounds activating irondependent,nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
2008;15:234-245 [PMID:18355723 DOI:10.1016/j.chembiol.2008.02.010]
6 Yagoda N,von Rechenberg M,Zaganjor E,Bauer AJ,Yang WS,Fridman DJ,Wolpaw AJ,Smukste I,Peltier JM,Boniface JJ,Smith R,Lessnick SL,Sahasrabudhe S,Stockwell BR.RAS-RAF-MEKdependent oxidative cell death involving voltage-dependent anion channels.
2007;447:864-868 [PMID:17568748 DOI:10.1038/nature05859]
7 Stockwell BR,Friedmann Angeli JP,Bayir H,Bush AI,Conrad M,Dixon SJ,Fulda S,Gascón S,Hatzios SK,Kagan VE,Noel K,Jiang X,Linkermann A,Murphy ME,Overholtzer M,Oyagi A,Pagnussat GC,Park J,Ran Q,Rosenfeld CS,Salnikow K,Tang D,Torti FM,Torti SV,Toyokuni S,Woerpel KA,Zhang DD.Ferroptosis:A Regulated Cell Death Nexus Linking Metabolism,Redox Biology,and Disease.
2017;171:273-285 [PMID:28985560 DOI:10.1016/j.cell.2017.09.021]
8 Tang M,Chen Z,Wu D,Chen L.Ferritinophagy/ferroptosis:Iron-related newcomers in human diseases.
2018;233:9179-9190 [PMID:30076709 DOI:10.1002/jcp.26954]
9 Liang C,Zhang X,Yang M,Dong X.Recent Progress in Ferroptosis Inducers for Cancer Therapy.
2019;31:e1904197 [PMID:31595562 DOI:10.1002/adma.201904197]
10 Hou W,Xie Y,Song X,Sun X,Lotze MT,Zeh HJ 3rd,Kang R,Tang D.Autophagy promotes ferroptosis by degradation of ferritin.
2016;12:1425-1428 [PMID:27245739 DOI:10.1080/15548627.2016.1187366]
11 Sun X,Ou Z,Chen R,Niu X,Chen D,Kang R,Tang D.Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells.
2016;63:173-184 [PMID:26403645 DOI:10.1002/hep.28251]
12 Doll S,Proneth B,Tyurina YY,Panzilius E,Kobayashi S,Ingold I,Irmler M,Beckers J,Aichler M,Walch A,Prokisch H,Trümbach D,Mao G,Qu F,Bayir H,Füllekrug J,Scheel CH,Wurst W,Schick JA,Kagan VE,Angeli JP,Conrad M.ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition.
2017;13:91-98 [PMID:27842070 DOI:10.1038/nchembio.2239]
13 Dixon SJ,Winter GE,Musavi LS,Lee ED,Snijder B,Rebsamen M,Superti-Furga G,Stockwell BR.Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death.
2015;10:1604-1609 [PMID:25965523 DOI:10.1021/acschembio.5b00245]
14 Anthonymuthu TS,Kenny EM,Shrivastava I,Tyurina YY,Hier ZE,Ting HC,Dar HH,Tyurin VA,Nesterova A,Amoscato AA,Mikulska-Ruminska K,Rosenbaum JC,Mao G,Zhao J,Conrad M,Kellum JA,Wenzel SE,VanDemark AP,Bahar I,Kagan VE,Bay?r H.Empowerment of 15-Lipoxygenase Catalytic Competence in Selective Oxidation of Membrane ETE-PE to Ferroptotic Death Signals,HpETE-PE.
2018;140:17835-17839 [PMID:30525572 DOI:10.1021/jacs.8b09913]
15 Song X,Zhu S,Chen P,Hou W,Wen Q,Liu J,Xie Y,Klionsky DJ,Kroemer G,Lotze MT,Zeh HJ,Kang R,Tang D.AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System X
Activity.
2018;28:2388-2399.e5 [PMID:30057310 DOI:10.1016/j.cub.2018.05.094]
16 Hao S,Liang B,Huang Q,Dong S,Wu Z,He W,Shi M.Metabolic networks in ferroptosis.
2018;15:5405-5411 [PMID:29556292 DOI:10.3892/ol.2018.8066]
17 Cao JY,Dixon SJ.Mechanisms of ferroptosis.
2016;73:2195-2209 [PMID:27048822 DOI:10.1007/s00018-016-2194-1]
18 Bersuker K,Hendricks JM,Li Z,Magtanong L,Ford B,Tang PH,Roberts MA,Tong B,Maimone TJ,Zoncu R,Bassik MC,Nomura DK,Dixon SJ,Olzmann JA.The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis.
2019;575:688-692 [PMID:31634900 DOI:10.1038/s41586-019-1705-2]
19 Torti SV,Torti FM.Iron and cancer:more ore to be mined.
2013;13:342-355[PMID:23594855 DOI:10.1038/nrc3495]
20 Yang WS,Stockwell BR.Ferroptosis:Death by Lipid Peroxidation.
2016;26:165-176 [PMID:26653790 DOI:10.1016/j.tcb.2015.10.014]
21 Wang S,Luo J,Zhang Z,Dong D,Shen Y,Fang Y,Hu L,Liu M,Dai C,Peng S,Fang Z,Shang P.Iron and magnetic:new research direction of the ferroptosis-based cancer therapy.
2018;8:1933-1946 [PMID:30416846]
22 Bogdan AR,Miyazawa M,Hashimoto K,Tsuji Y.Regulators of Iron Homeostasis:New Players in Metabolism,Cell Death,and Disease.
2016;41:274-286 [PMID:26725301 DOI:10.1016/j.tibs.2015.11.012]
23 Manz DH,Blanchette NL,Paul BT,Torti FM,Torti SV.Iron and cancer:recent insights.
2016;1368:149-161 [PMID:26890363 DOI:10.1111/nyas.13008]
24 Kindrat I,Tryndyak V,de Conti A,Shpyleva S,Mudalige TK,Kobets T,Erstenyuk AM,Beland FA,Pogribny IP.MicroRNA-152-mediated dysregulation of hepatic transferrin receptor 1 in liver carcinogenesis.
2016;7:1276-1287 [PMID:26657500 DOI:10.18632/oncotarget.6004]
25 Geng N,Shi BJ,Li SL,Zhong ZY,Li YC,Xua WL,Zhou H,Cai JH.Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells.
2018;22:3826-3836 [PMID:29949159 DOI:10.26355/eurrev_201806_15267]
26 Yang WS,Kim KJ,Gaschler MM,Patel M,Shchepinov MS,Stockwell BR.Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis.
2016;113:E4966-E4975 [PMID:27506793 DOI:10.1073/pnas.1603244113]
27 Du J,Wang T,Li Y,Zhou Y,Wang X,Yu X,Ren X,An Y,Wu Y,Sun W,Fan W,Zhu Q,Wang Y,Tong X.DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin.
2019;131:356-369 [PMID:30557609 DOI:10.1016/j.freeradbiomed.2018.12.011]
28 Zhou B,Liu J,Kang R,Klionsky DJ,Kroemer G,Tang D.Ferroptosis is a type of autophagydependent cell death.
2020;66:89-100 [PMID:30880243 DOI:10.1016/j.semcancer.2019.03.002]
29 Sun X,Ou Z,Xie M,Kang R,Fan Y,Niu X,Wang H,Cao L,Tang D.HSPB1 as a novel regulator of ferroptotic cancer cell death.
2015;34:5617-5625 [PMID:25728673 DOI:10.1038/onc.2015.32]
30 Yuan H,Li X,Zhang X,Kang R,Tang D.CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation.
2016;478:838-844 [PMID:27510639 DOI:10.1016/j.bbrc.2016.08.034]
31 Dixon SJ,Stockwell BR.The role of iron and reactive oxygen species in cell death.
2014;10:9-17 [PMID:24346035 DOI:10.1038/nchembio.1416]
32 Parisi LR,Li N,Atilla-Gokcumen GE.Very Long Chain Fatty Acids Are Functionally Involved in Necroptosis.
2017;24:1445-1454.e8 [PMID:29033315 DOI:10.1016/j.chembiol.2017.08.026]
33 Magtanong L,Ko PJ,To M,Cao JY,Forcina GC,Tarangelo A,Ward CC,Cho K,Patti GJ,Nomura DK,Olzmann JA,Dixon SJ.Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State.
2019;26:420-432.e9 [PMID:30686757 DOI:10.1016/j.chembiol.2018.11.016]
34 Conrad M,Kagan VE,Bayir H,Pagnussat GC,Head B,Traber MG,Stockwell BR.Regulation of lipid peroxidation and ferroptosis in diverse species.
2018;32:602-619 [PMID:29802123 DOI:10.1101/gad.314674.118]
35 Zhong H,Yin H.Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer:focusing on mitochondria.
2015;4:193-199 [PMID:25598486 DOI:10.1016/j.redox.2014.12.011]
36 Shimbara-Matsubayashi S,Kuwata H,Tanaka N,Kato M,Hara S.Analysis on the Substrate Specificity of Recombinant Human Acyl-CoA Synthetase ACSL4 Variants.
2019;42:850-855 [PMID:31061331 DOI:10.1248/bpb.b19-00085]
37 Imai H,Matsuoka M,Kumagai T,Sakamoto T,Koumura T.Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.
2017;403:143-170[PMID:28204974 DOI:10.1007/82_2016_508]
38 Zhao J,O'Donnell VB,Balzar S,St Croix CM,Trudeau JB,Wenzel SE.15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells.
2011;108:14246-14251 [PMID:21831839 DOI:10.1073/pnas.1018075108]
39 Forman HJ,Zhang H,Rinna A.Glutathione:overview of its protective roles,measurement,and biosynthesis.
2009;30:1-12 [PMID:18796312 DOI:10.1016/j.mam.2008.08.006]
40 Lv H,Zhen C,Liu J,Yang P,Hu L,Shang P.Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy.
2019;2019:3150145[PMID:31281572 DOI:10.1155/2019/3150145]
41 Liu J,Xia X,Huang P.xCT:A Critical Molecule That Links Cancer Metabolism to Redox Signaling.
2020;28:2358-2366 [PMID:32931751 DOI:10.1016/j.ymthe.2020.08.021]
42 Koppula P,Zhuang L,Gan B.Cystine transporter SLC7A11/xCT in cancer:ferroptosis,nutrient dependency,and cancer therapy.
2021;12:599-620 [PMID:33000412 DOI:10.1007/s13238-020-00789-5]
43 Ottestad-Hansen S,Hu QX,Follin-Arbelet VV,Bentea E,Sato H,Massie A,Zhou Y,Danbolt NC.The cystine-glutamate exchanger (xCT,Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain.
2018;66:951-970 [PMID:29350434 DOI:10.1002/glia.23294]
44 Miyamoto K,Watanabe M,Boku S,Sukeno M,Morita M,Kondo H,Sakaguchi K,Taguchi T,Sakai T.xCT Inhibition Increases Sensitivity to Vorinostat in a ROS-Dependent Manner.
2020;12 [PMID:32235498 DOI:10.3390/cancers12040827]
45 Xie Y,Li J,Kang R,Tang D.Interplay Between Lipid Metabolism and Autophagy.
2020;8:431 [PMID:32582708 DOI:10.3389/fcell.2020.00431]
46 Hayano M,Yang WS,Corn CK,Pagano NC,Stockwell BR.Loss of cysteinyl-tRNA synthetase(CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation.
2016;23:270-278 [PMID:26184909 DOI:10.1038/cdd.2015.93]
47 Kang R,Kroemer G,Tang D.The tumor suppressor protein p53 and the ferroptosis network.
2019;133:162-168 [PMID:29800655 DOI:10.1016/j.freeradbiomed.2018.05.074]
48 Jennis M,Kung CP,Basu S,Budina-Kolomets A,Leu JI,Khaku S,Scott JP,Cai KQ,Campbell MR,Porter DK,Wang X,Bell DA,Li X,Garlick DS,Liu Q,Hollstein M,George DL,Murphy ME.An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model.
2016;30:918-930 [PMID:27034505 DOI:10.1101/gad.275891.115]
49 Xie Y,Zhu S,Song X,Sun X,Fan Y,Liu J,Zhong M,Yuan H,Zhang L,Billiar TR,Lotze MT,Zeh HJ 3rd,Kang R,Kroemer G,Tang D.The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity.
2017;20:1692-1704 [PMID:28813679 DOI:10.1016/j.celrep.2017.07.055]
50 Li Q,Han X,Lan X,Gao Y,Wan J,Durham F,Cheng T,Yang J,Wang Z,Jiang C,Ying M,Koehler RC,Stockwell BR,Wang J.Inhibition of neuronal ferroptosis protects hemorrhagic brain.
2017;2:e90777 [PMID:28405617 DOI:10.1172/jci.insight.90777]
51 Ooko E,Saeed ME,Kadioglu O,Sarvi S,Colak M,Elmasaoudi K,Janah R,Greten HJ,Efferth T.Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells.
2015;22:1045-1054 [PMID:26407947 DOI:10.1016/j.phymed.2015.08.002]
52 Gong Y,Wang N,Liu N,Dong H.Lipid Peroxidation and GPX4 Inhibition Are Common Causes for Myofibroblast Differentiation and Ferroptosis.
2019;38:725-733 [PMID:31140862 DOI:10.1089/dna.2018.4541]
53 Doll S,Freitas FP,Shah R,Aldrovandi M,da Silva MC,Ingold I,Goya Grocin A,Xavier da Silva TN,Panzilius E,Scheel CH,Mour?o A,Buday K,Sato M,Wanninger J,Vignane T,Mohana V,Rehberg M,Flatley A,Schepers A,Kurz A,White D,Sauer M,Sattler M,Tate EW,Schmitz W,Schulze A,O'Donnell V,Proneth B,Popowicz GM,Pratt DA,Angeli JPF,Conrad M.FSP1 is a glutathione-independent ferroptosis suppressor.
2019;575:693-698 [PMID:31634899 DOI:10.1038/s41586-019-1707-0]
54 Mazure NM.VDAC in cancer.
2017;1858:665-673 [PMID:28283400 DOI:10.1016/j.bbabio.2017.03.002]
55 Maldonado EN.VDAC-Tubulin,an Anti-Warburg Pro-Oxidant Switch.
2017;7:4[PMID:28168164 DOI:10.3389/fonc.2017.00004]
56 Chen Y,Liu Y,Lan T,Qin W,Zhu Y,Qin K,Gao J,Wang H,Hou X,Chen N,Friedmann Angeli JP,Conrad M,Wang C.Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe.
2018;140:4712-4720 [PMID:29569437 DOI:10.1021/jacs.8b01462]
57 Lemasters JJ.Evolution of Voltage-Dependent Anion Channel Function:From Molecular Sieve to Governator to Actuator of Ferroptosis.
2017;7:303 [PMID:29312883 DOI:10.3389/fonc.2017.00303]
58 Arya N,Wyse JM,Jayaraman S,Ball CG,Lam E,Paquin SC,Lightfoot P,Sahai AV.A proposal for the ideal algorithm for the diagnosis,staging,and treatment of pancreas masses suspicious for pancreatic adenocarcinoma:Results of a working group of the Canadian Society for Endoscopic Ultrasound.
2020;9:154-161 [PMID:32584310]
59 Tang R,Hua J,Xu J,Liang C,Meng Q,Liu J,Zhang B,Yu X,Shi S.The role of ferroptosis regulators in the prognosis,immune activity and gemcitabine resistance of pancreatic cancer.
2020;8:1347 [PMID:33313092 DOI:10.21037/atm-20-2554a]
60 Zhu S,Zhang Q,Sun X,Zeh HJ 3rd,Lotze MT,Kang R,Tang D.HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells.
2017;77:2064-2077 [PMID:28130223 DOI:10.1158/0008-5472.CAN-16-1979]
61 Shintoku R,Takigawa Y,Yamada K,Kubota C,Yoshimoto Y,Takeuchi T,Koshiishi I,Torii S.Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3.
2017;108:2187-2194 [PMID:28837253 DOI:10.1111/cas.13380]
62 Kuang Y,Sechi M,Nurra S,Ljungman M,Neamati N.Design and Synthesis of Novel Reactive Oxygen Species Inducers for the Treatment of Pancreatic Ductal Adenocarcinoma.
2018;61:1576-1594 [PMID:29328656 DOI:10.1021/acs.jmedchem.7b01463]
63 Kasukabe T,Honma Y,Okabe-Kado J,Higuchi Y,Kato N,Kumakura S.Combined treatment with cotylenin A and phenethyl isothiocyanate induces strong antitumor activity mainly through the induction of ferroptotic cell death in human pancreatic cancer cells.
2016;36:968-976[PMID:27375275 DOI:10.3892/or.2016.4867]
64 Yamaguchi Y,Kasukabe T,Kumakura S.Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis.
2018;52:1011-1022[PMID:29393418 DOI:10.3892/ijo.2018.4259]
65 Eling N,Reuter L,Hazin J,Hamacher-Brady A,Brady NR.Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells.
2015;2:517-532 [PMID:26097885 DOI:10.18632/oncoscience.160]
66 Wang K,Zhang Z,Wang M,Cao X,Qi J,Wang D,Gong A,Zhu H.Role of GRP78 inhibiting artesunate-induced ferroptosis in
mutant pancreatic cancer cells.
2019;13:2135-2144 [PMID:31456633 DOI:10.2147/DDDT.S199459]
67 Badgley MA,Kremer DM,Maurer HC,DelGiorno KE,Lee HJ,Purohit V,Sagalovskiy IR,Ma A,Kapilian J,Firl CEM,Decker AR,Sastra SA,Palermo CF,Andrade LR,Sajjakulnukit P,Zhang L,Tolstyka ZP,Hirschhorn T,Lamb C,Liu T,Gu W,Seeley ES,Stone E,Georgiou G,Manor U,Iuga A,Wahl GM,Stockwell BR,Lyssiotis CA,Olive KP.Cysteine depletion induces pancreatic tumor ferroptosis in mice.
2020;368:85-89 [PMID:32241947 DOI:10.1126/science.aaw9872]
68 Dai E,Han L,Liu J,Xie Y,Kroemer G,Klionsky DJ,Zeh HJ,Kang R,Wang J,Tang D.Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization
release and uptake of oncogenic KRAS protein.
2020;16:2069-2083 [PMID:31920150 DOI:10.1080/15548627.2020.1714209]
69 Mohamed AA,Omar AAA,El-Awady RR,Hassan SMA,Eitah WMS,Ahmed R,Khater A,Tantawi OMS,Mohamed AA.MiR-155 and MiR-665 Role as Potential Non-invasive Biomarkers for Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection.
2020;8:32-40 [PMID:32435610 DOI:10.2478/jtim-2020-0006]
70 Shan Y,Yang G,Huang H,Zhou Y,Hu X,Lu Q,Guo P,Hou J,Cao L,Tian F,Pan Q.Ubiquitin-Like Modifier Activating Enzyme 1 as a Novel Diagnostic and Prognostic Indicator That Correlates With Ferroptosis and the Malignant Phenotypes of Liver Cancer Cells.
2020;10:592413 [PMID:33344241 DOI:10.3389/fonc.2020.592413]
71 Liang JY,Wang DS,Lin HC,Chen XX,Yang H,Zheng Y,Li YH.A Novel Ferroptosis-related Gene Signature for Overall Survival Prediction in Patients with Hepatocellular Carcinoma.
2020;16:2430-2441 [PMID:32760210 DOI:10.7150/ijbs.45050]
72 Louandre C,Marcq I,Bouhlal H,Lachaier E,Godin C,Saidak Z,Fran?ois C,Chatelain D,Debuysscher V,Barbare JC,Chauffert B,Galmiche A.The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells.
2015;356:971-977 [PMID:25444922 DOI:10.1016/j.canlet.2014.11.014]
73 Zhang Z,Yao Z,Wang L,Ding H,Shao J,Chen A,Zhang F,Zheng S.Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells.
2018;14:2083-2103 [PMID:30081711 DOI:10.1080/15548627.2018.1503146]
74 Qi W,Li Z,Xia L,Dai J,Zhang Q,Wu C,Xu S.LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells.
2019;9:16185 [PMID:31700067 DOI:10.1038/s41598-019-52837-8]
75 Ndiaye H,Liu JY,Hall A,Minogue S,Morgan MY,Waugh MG.Immunohistochemical staining reveals differential expression of ACSL3 and ACSL4 in hepatocellular carcinoma and hepatic gastrointestinal metastases.
2020;40 [PMID:32286604 DOI:10.1042/bsr20200219]
76 Sun X,Niu X,Chen R,He W,Chen D,Kang R,Tang D.Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis.
2016;64:488-500 [PMID:27015352 DOI:10.1002/hep.28574]
77 Bai T,Wang S,Zhao Y,Zhu R,Wang W,Sun Y.Haloperidol,a sigma receptor 1 antagonist,promotes ferroptosis in hepatocellular carcinoma cells.
2017;491:919-925 [PMID:28756230 DOI:10.1016/j.bbrc.2017.07.136]
78 Bai T,Lei P,Zhou H,Liang R,Zhu R,Wang W,Zhou L,Sun Y.Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells.
2019;23:7349-7359 [PMID:31507082 DOI:10.1111/jcmm.14594]
79 Wang K,Zhang Z,Tsai HI,Liu Y,Gao J,Wang M,Song L,Cao X,Xu Z,Chen H,Gong A,Wang D,Cheng F,Zhu H.Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells.
2021;28:1222-1236 [PMID:33097833 DOI:10.1038/s41418-020-00644-4]
80 Ou W,Mulik RS,Anwar A,McDonald JG,He X,Corbin IR.Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma.
2017;112:597-607 [PMID:28893626 DOI:10.1016/j.freeradbiomed.2017.09.002]
81 Shang Y,Luo M,Yao F,Wang S,Yuan Z,Yang Y.Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells.
2020;72:109633 [PMID:32283255 DOI:10.1016/j.cellsig.2020.109633]
82 Li ZJ,Dai HQ,Huang XW,Feng J,Deng JH,Wang ZX,Yang XM,Liu YJ,Wu Y,Chen PH,Shi H,Wang JG,Zhou J,Lu GD.Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma.
2021;42:301-310 [PMID:32699265 DOI:10.1038/s41401-020-0478-3]
83 Tang H,Chen D,Li C,Zheng C,Wu X,Zhang Y,Song Q,Fei W.Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells.
2019;572:118782 [PMID:31678528 DOI:10.1016/j.ijpharm.2019.118782]
84 Wang G,Liu X,Wang S,Ge N,Guo J,Sun S.Endoscopic Ultrasound-guided Gastroenterostomy:A Promising Alternative to Surgery.
2019;7:93-99 [PMID:31637179 DOI:10.2478/jtim-2019-0021]
85 Lee JY,Nam M,Son HY,Hyun K,Jang SY,Kim JW,Kim MW,Jung Y,Jang E,Yoon SJ,Kim J,Seo J,Min JK,Oh KJ,Han BS,Kim WK,Bae KH,Song J,Huh YM,Hwang GS,Lee EW,Lee SC.Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer.
2020;117:32433-32442 [PMID:33288688 DOI:10.1073/pnas.2006828117]
86 Wang C,Shi M,Ji J,Cai Q,Zhao Q,Jiang J,Liu J,Zhang H,Zhu Z,Zhang J.Stearoyl-CoA desaturase 1 (SCD1) facilitates the growth and anti-ferroptosis of gastric cancer cells and predicts poor prognosis of gastric cancer.
2020;12:15374-15391 [PMID:32726752 DOI:10.18632/aging.103598]
87 Hao S,Yu J,He W,Huang Q,Zhao Y,Liang B,Zhang S,Wen Z,Dong S,Rao J,Liao W,Shi M.Cysteine Dioxygenase 1 Mediates Erastin-Induced Ferroptosis in Human Gastric Cancer Cells.
2017;19:1022-1032 [PMID:29144989 DOI:10.1016/j.neo.2017.10.005]
88 Sun X,Yang S,Feng X,Zheng Y,Zhou J,Wang H,Zhang Y,Sun H,He C.The modification of ferroptosis and abnormal lipometabolism through overexpression and knockdown of potential prognostic biomarker perilipin2 in gastric carcinoma.
2020;23:241-259 [PMID:31520166 DOI:10.1007/s10120-019-01004-z]
89 Gao Z,Deng G,Li Y,Huang H,Sun X,Shi H,Yao X,Gao L,Ju Y,Luo M.Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis,ferroptosis activation and mesenchymal phenotype suppression.
2020;126:110092[PMID:32203890 DOI:10.1016/j.biopha.2020.110092]
90 Guan Z,Chen J,Li X,Dong N.Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation.
2020;40 [PMID:32776119 DOI:10.1042/bsr20201807]
91 Nunes G,Marques PP,Patita M,Allen M,Gargaté L.EUS-guided recanalization of complete colorectal anastomotic stenosis using a lumen-apposing metal stent.
2019;8:211-212 [PMID:30785118 DOI:10.4103/eus.eus_62_18]
92 Sak K.A Hypothetical Approach on Gender Differences in Cancer Diagnosis.
2019;7:90-92 [PMID:31637178 DOI:10.2478/jtim-2019-0020]
93 Sui X,Zhang R,Liu S,Duan T,Zhai L,Zhang M,Han X,Xiang Y,Huang X,Lin H,Xie T.RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer.
2018;9:1371 [PMID:30524291 DOI:10.3389/fphar.2018.01371]
94 Lu D,Yang Z,Xia Q,Gao S,Sun S,Luo X,Li Z,Zhang X,Li X.ACADSB regulates ferroptosis and affects the migration,invasion,and proliferation of colorectal cancer cells.
2020;44:2334-2343 [PMID:32776663 DOI:10.1002/cbin.11443]
95 Park S,Oh J,Kim M,Jin EJ.Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis.
2018;22:334-340 [PMID:30460115 DOI:10.1080/19768354.2018.1512521]
96 Xia Y,Liu S,Li C,Ai Z,Shen W,Ren W,Yang X.Discovery of a novel ferroptosis inducertalaroconvolutin A-killing colorectal cancer cells
and in vivo.
2020;11:988[PMID:33203867 DOI:10.1038/s41419-020-03194-2]
97 Zhang L,Liu W,Liu F,Wang Q,Song M,Yu Q,Tang K,Teng T,Wu D,Wang X,Han W,Li Y.IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer.
2020;2020:1675613 [PMID:32322334 DOI:10.1155/2020/1675613]
98 Shen LD,Qi WH,Bai JJ,Zuo CY,Bai DL,Gao WD,Zong XL,Hao TT,Ma Y,Cao GC.Resibufogenin inhibited colorectal cancer cell growth and tumorigenesis through triggering ferroptosis and ROS production mediated by GPX4 inactivation.
2021;304:313-322 [PMID:31961485 DOI:10.1002/ar.24378]
99 Sharma P,Shimura T,Banwait JK,Goel A.Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer.
2020;41:1385-1394 [PMID:32835374 DOI:10.1093/carcin/bgaa090]
100 Chen P,Li X,Zhang R,Liu S,Xiang Y,Zhang M,Chen X,Pan T,Yan L,Feng J,Duan T,Wang D,Chen B,Jin T,Wang W,Chen L,Huang X,Zhang W,Sun Y,Li G,Kong L,Li Y,Yang Z,Zhang Q,Zhuo L,Sui X,Xie T.Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation.
2020;10:5107-5119 [PMID:32308771 DOI:10.7150/thno.44705]
101 Lorenzato A,Magrì A,Matafora V,Audrito V,Arcella P,Lazzari L,Montone M,Lamba S,Deaglio S,Siena S,Bertotti A,Trusolino L,Bachi A,Di Nicolantonio F,Bardelli A,Arena S.Vitamin C Restricts the Emergence of Acquired Resistance to EGFR-Targeted Therapies in Colorectal Cancer.
2020;12 [PMID:32183295 DOI:10.3390/cancers12030685]
102 Friedmann Angeli JP,Krysko DV,Conrad M.Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion.
2019;19:405-414 [PMID:31101865 DOI:10.1038/s41568-019-0149-1]
103 Yang WS,SriRamaratnam R,Welsch ME,Shimada K,Skouta R,Viswanathan VS,Cheah JH,Clemons PA,Shamji AF,Clish CB,Brown LM,Girotti AW,Cornish VW,Schreiber SL,Stockwell BR.Regulation of ferroptotic cancer cell death by GPX4.
2014;156:317-331 [PMID:24439385 DOI:10.1016/j.cell.2013.12.010]
104 Zhang Y,Tan H,Daniels JD,Zandkarimi F,Liu H,Brown LM,Uchida K,O'Connor OA,Stockwell BR.Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model.
2019;26:623-633.e9 [PMID:30799221 DOI:10.1016/j.chembiol.2019.01.008]
105 Gout PW,Buckley AR,Simms CR,Bruchovsky N.Sulfasalazine,a potent suppressor of lymphoma growth by inhibition of the x(c)-cystine transporter:a new action for an old drug.
2001;15:1633-1640 [PMID:11587223 DOI:10.1038/sj.leu.2402238]
106 Kim EH,Shin D,Lee J,Jung AR,Roh JL.CISD2 inhibition overcomes resistance to sulfasalazineinduced ferroptotic cell death in head and neck cancer.
2018;432:180-190 [PMID:29928961 DOI:10.1016/j.canlet.2018.06.018]
107 Dixon SJ,Patel DN,Welsch M,Skouta R,Lee ED,Hayano M,Thomas AG,Gleason CE,Tatonetti NP,Slusher BS,Stockwell BR.Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis.
2014;3:e02523 [PMID:24844246 DOI:10.7554/eLife.02523]
108 Shimada K,Skouta R,Kaplan A,Yang WS,Hayano M,Dixon SJ,Brown LM,Valenzuela CA,Wolpaw AJ,Stockwell BR.Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis.
2016;12:497-503 [PMID:27159577 DOI:10.1038/nchembio.2079]
109 Guo J,Xu B,Han Q,Zhou H,Xia Y,Gong C,Dai X,Li Z,Wu G.Ferroptosis:A Novel Anti-tumor Action for Cisplatin.
2018;50:445-460 [PMID:28494534 DOI:10.4143/crt.2016.572]
110 Abrams RP,Carroll WL,Woerpel KA.Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells.
2016;11:1305-1312 [PMID:26797166 DOI:10.1021/acschembio.5b00900]
111 NaveenKumar SK,SharathBabu BN,Hemshekhar M,Kemparaju K,Girish KS,Mugesh G.The Role of Reactive Oxygen Species and Ferroptosis in Heme-Mediated Activation of Human Platelets.
2018;13:1996-2002 [PMID:29869870 DOI:10.1021/acschembio.8b00458]
112 Ma S,Henson ES,Chen Y,Gibson SB.Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells.
2016;7:e2307 [PMID:27441659 DOI:10.1038/cddis.2016.208]
113 Yuan H,Li X,Zhang X,Kang R,Tang D.Identification of ACSL4 as a biomarker and contributor of ferroptosis.
2016;478:1338-1343 [PMID:27565726 DOI:10.1016/j.bbrc.2016.08.124]
114 Shi F,Zhang P,Mao Y,Wang C,Zheng M,Zhao Z.The nitroxide Tempo inhibits hydroxyl radical production from the Fenton-like reaction of iron(II)-citrate with hydrogen peroxide.
2017;483:159-164 [PMID:28042034 DOI:10.1016/j.bbrc.2016.12.174]
115 Guerrero-Hue M,García-Caballero C,Palomino-Antolín A,Rubio-Navarro A,Vázquez-Carballo C,Herencia C,Martín-Sanchez D,Farré-Alins V,Egea J,Cannata P,Praga M,Ortiz A,Egido J,Sanz AB,Moreno JA.Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death.
2019;33:8961-8975 [PMID:31034781 DOI:10.1096/fj.201900077R]
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
