Effects of Helicobacter pylori infection in gastrointestinal tract malignant diseases:From the oral cavity to rectum
INTRODUCTION
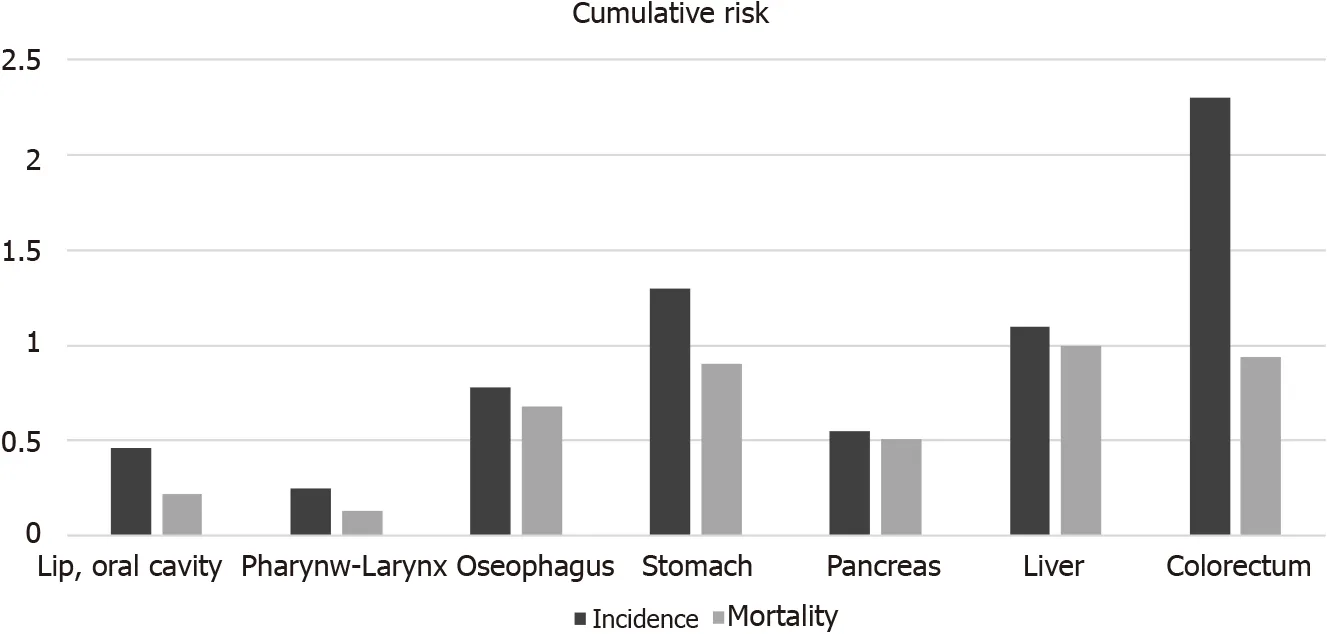
According to 2020 Global Cancer Observatory data,seven of the top twenty cancers with the highest cumulative risk of incidence affect the gastrointestinal system,including oral cavity,laryngeal-pharynx,esophagus,stomach,pancreas,liver and colorectal cancers (CRC).In 2020,gastrointestinal oncology diseases accounted for greater than 26% of all cancers worldwide.With the exception of pancreatic cancer and liver cancer,other cancer tracts were interlinked (Figure 1)[1].Three major risk factors are thought to be related to cancers:Obesity,infection and ultraviolet radiation.Several infections are considered to be related to cancer formation,including infection by
(
),human papillomavirus (HPV),and hepatitis B and C viruses,and these different infectious agents account for greater than 90% of infectionrelated cancers worldwide[2].
was identified as an origin of peptic ulcer disease and has become an important public health issue worldwide since 1982.With different geographic areas,ages,ethnicities and socioeconomic statuses,the prevalence rates of
infection are also different[3,4].Approximately 50% of people worldwide are infected by this bacterium.At the beginning of the 21
century,the prevalence was reduced in highly industrialized countries of the Western world.In contrast,the prevalence remains high in developing and newly industrialized countries.The discrepancy in prevalence may result from the degree of urbanization,sanitation,access to clean water,and socioeconomic status[5].
According to the International Agency for Research on Cancer,
is a human carcinogen highly correlated with gastric cancer (GC)[6].
also accounted for approximately 810000 infection-related cancer cases,which is greater than that reported for any other microorganism,in 2018[2].
Because
exhibits oral-oral or fecal-oral transmission and the whole gastrointestinal tract is connected,we further surveyed the role of
in different gastrointestinal malignant diseases to provide a better understanding of the relationship between
infection and malignant diseases.GC was highly correlated to
infection,but the relationship between
and cancer formation in other gastrointestinal tract malignant diseases,such as oral cavity cancer,laryngeal cancer,esophageal cancer,and colon cancer,has not been completely studied.Recent studies have shown some association between GC and
infection but lack a further overview of
infection and malignant diseases of the whole gastrointestinal tract.Past studies used the host viewpoint and examined which pathogen could cause disease in the host.This review article used the viewpoint of microorganisms and focused on the effects of
infection in gastrointestinal tract malignant diseases from the oral cavity to the rectum to further realize the connection between infectious and malignant diseases.
On Christmas Eve I saw that my mother had outdone herself in creating a strange menu. She was pulling black veins1 out of the backs of fleshy prawns2. The kitchen was littered with appalling3 mounds4 of raw food: A slimy rock cod5 with bulging6 eyes that pleaded not to be thrown into a pan of hot oil. Tofu, which looked like stacked wedges of rubbery white sponges. A bowl soaking dried fungus7 back to life. A plate of squid, their back crisscrossed with knife markings so they resembled bicycle tires.
ORAL CANCER
Epidemiology of oral cancer
Oral cancer represents approximately 3% of all cancers worldwide and is the 6
most common cancer globally.As a popular habit in Asian countries,betel quid chewing is associated with periodontal disease,oral submucous fibrosis,and oral cancer[7,8].Compared with other tumors in the oral cavity,oral squamous cell carcinoma (OSCC)tends to exhibit local invasion and metastasis[9].In addition,OSCC occurs more frequently in middle-aged and older populations,particularly in men[10].
Pathological differences in oral cancer
Constituting 94% of oral malignancies,OSCC is far more common than the remaining malignancies,including salivary gland cancer,soft tissue sarcoma,jaw osteosarcoma,non-Hodgkin’s lymphoma,melanomas and metastatic tumors,in the oral cavity[11,12].
Role of H.pylori in oral cancer
As a Class I carcinogen,the role of
in oral cancer is not yet clear.Whether the colonization of
is facilitated by betel chewing-related lesions or the resulting chemical changes in the oral cavity remains an important issue to be studied.By comparing the prevalence of
in patients with oral cancer and healthy controls with different betel chewing statuses (Table 1),Fernando
[13] noticed a significantly higher rate of infection among betel chewers regardless of the cancer status.Thus,betel chewing,not oral cancer,is a potential contributing factor to
infection.
Few studies have shown the association between
and oral cancer.Grandis
[14] reported a similar seroprevalence of
in 21 patients with oral cancer and 21 controls;thus,the association could not be proven.Another study adopted polymerase chain reaction (PCR) and culture techniques to identify the existence of
in serum and tissue samples and reported insignificant differences in the prevalence of
between patients with oral cancer and controls.Nevertheless,the odds ratio (OR) was 3.0 [95% confidence interval (CI):0.34-26.4] by culture and 1.5(95%CI:0.28-8.0) by PCR[15].Only a few studies have attempted to examine the presence of
in OSCC[16].Due to conflicting results,the relationship between
and OSCC cannot be concluded.The variable results may be caused by differences in methodology,specifically the disparity in the sensitivity and specificity of diagnostic methods.Using the three detection methods [
immunoglobin (Ig)G antibodies,PCR,and histochemical staining],Meng
[17] suggested an inverse association between
infection and OSCC in the subgroup of individuals over 60 years of age according to the prevalence (35.3%
54.8%,
=0.012),stratification analysis (
=0.037) and Spearman's correlation (coef.=-0.191,
=0.012).Regardless of race,lifestyle and habitual risk factors,the absence of
in the available OSCC cohorts indicates that
is unlikely to contribute to OSCC pathogenesis[18].
Role of the host effect in oral cancer
Three days he rode, till he came to the green plain whence the three ways started, and read the words carved on tile great stone that stood there. I may not take the left road, lest I die, he thought, nor the middle road, lest I know hunger and cold. Rather will I take the right-hand road, whereon, though my poor horse perish, I at least shall keep my life. So he reined22 to the right.
Summary
According to currently available studies,the relationship between
and oral malignancy cannot be made at present (Tables 1 and 2).Results varied among the studies due to the use of different diagnostic methods (culture,immunohistochemistry,enzyme-linked immunosorbent assay,PCR) adopted for
identification.Overall,the meta-analysis revealed a nonsignificant association between the bacterium and OSCC.
So they caused Placida to seem to have a violent fever, and Vivien to languish140 and grow dull, and made each of them very uneasy about the other, and then, finding a moment when they were apart, the Fairy Mirlifiche suddenly appeared to Placida, and said-- I have just seen Prince Vivien, and he seemed to me to be very ill
PHARYNGEAL-LARYNGEAL CANCER
Epidemiology of pharyngeal-laryngeal cancer
Although it is steadily decreasing in incidence,GC remains one of the most common malignant diseases worldwide[35].According to GLOBOCAN 2020 data,GC is the sixth most commonly diagnosed cancer and the fifth leading cause of cancer mortality in the world,following lung,breast,colorectal and liver cancer.A Global Cancer Observatory report in 2018 noted that the cumulative risk of GC was higher in men than in women (1.87% and 0.79%)[1].Compared to North and East Africa and North America,the incidence of GC was higher in East and Central Asia.In East Asia,the average incidence of GC for men and women is 3.21 and 1.32 per individual,respectively,whereas the incidence is 0.56 per million individuals in North America.The risk varies from six-to fifteen-fold between areas with the highest and the lowest incidence.The cause of this difference might be related to region and culture[56].Ninety-five percent of GCs are adenocarcinomas followed by primary gastric lymphoma,and we focus on reviewing adenocarcinoma in this section.According to the anatomical site,gastric adenocarcinomas can be classified into cardia GCs and noncardia GCs.The pathogenesis of cardia GCs might be related to GERD or EAC.Noncardia GCs are caused by
-related atrophic gastritis and a variety of environmental factors,such as diet,alcohol,and smoking[57].
Pathological differences in pharyngeal-laryngeal cancer
Most of these cancers are squamous cell carcinoma,accounting for 85%-95% of pharyngeal-laryngeal malignancies[27].
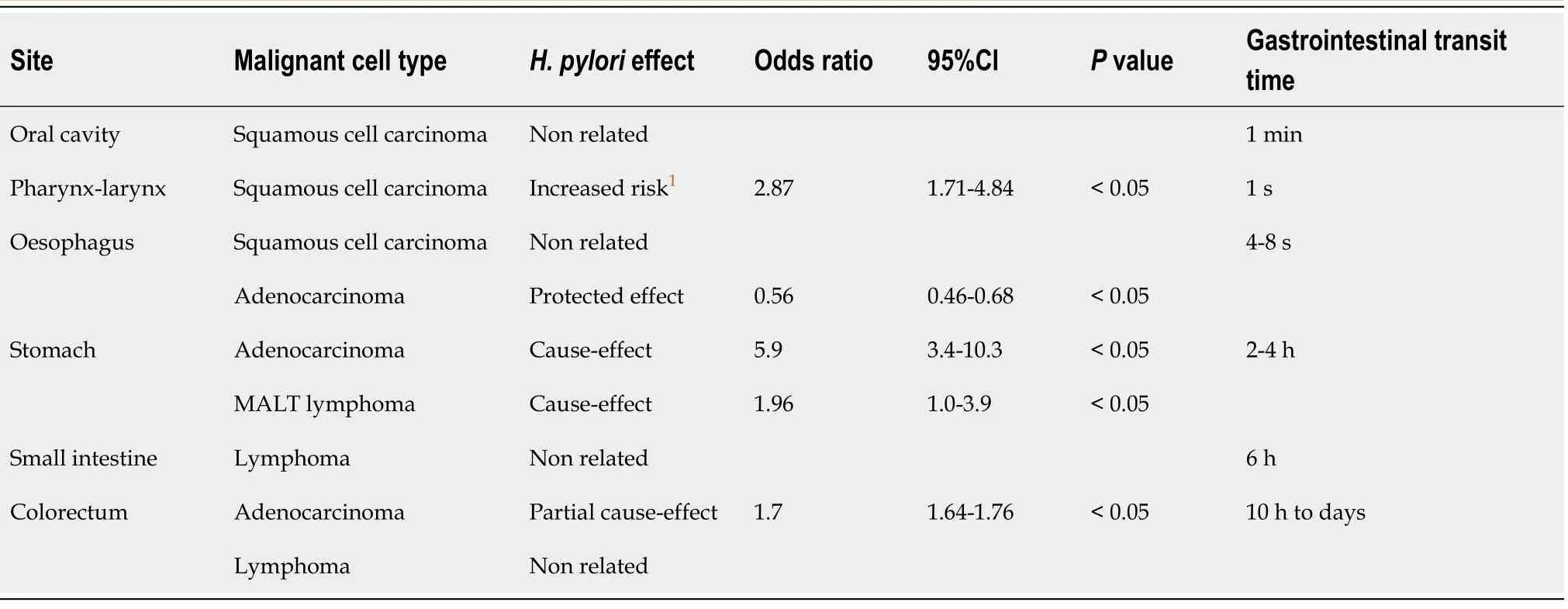
Role of H.pylori in pharyngeal-laryngeal cancer
has been detected in tooth plaque,saliva,nasal sinuses,and the middle ear[28,29].The association between
infection and pharyngeal-laryngeal cancer has been described by Zhou
[30].Eleven studies were included in a meta-analysis that demonstrated a significantly higher rate of
infection in patients with pharyngeal-laryngeal cancer compared with healthy controls (OR=2.87,95%CI:1.71-4.84;
<0.0001).Furthermore,the ORs for laryngeal carcinoma were greater than those for pharyngeal cancer [(OR:3.28,95%CI:1.91-5.63)
(OR:1.35,95%CI:0.86-2.12),respectively].On the basis of the study results,a relationship between
infection and laryngeal carcinoma but not pharyngeal cancer was suggested.This association may result from the direct exposure of the larynx to known carcinogens (
alcohol and tobacco),whereas mucosal and immune barriers were broken down after
infected the larynx.In patients with either benign or malignant laryngeal diseases,
was detected in greater than one-third (38.8%) of the biopsy samples from the larynx.The infection rate of
was highest in patients with laryngeal cancer (46.2%) and chronic laryngitis (45.5%) and was significantly lower in controls(9.1%)[31].Based on the results of a meta-analysis,
infection increases the risk of laryngeal cancer by twofold compared to controls[32].
It is well known that
modifies the host’s immune response,resulting in GC.A similar mechanism might contribute to oral carcinoma;however,this relationship has not been revealed to date.To illustrate the potential relationship between
and oral cancer,a prospective cohort should be conducted in the future.Other risk factors,such as smoking,alcohol consumption,fungi (candidiasis) and viruses (Epstein-Barr virus and HPV),have already been extensively studied[19].
In the past fifty years,the incidence of GC has steadily declined.This trend was more significant in East Asia and might be due to a successful reduction in the number of
infections.Approximately 90% of cases of non-cardia GCs are attributable to
infection.Given
eradication and reduced infection rates,the incidence of non-cardia GCs is also declining[58].In addition to
eradication,improved food conservation,higher standards of hygiene,and high intake of fresh fruits and vegetables could explain the reduced incidence of GCs[59].
In addition,acting as confounders,smoking cigarettes and alcohol consumption could mask the true relationship between laryngeal cancer and
infection,and well examined evidence supports the role of these confounders in the development of laryngeal cancer.Zhou
[30] declared that no adjustment was made to eliminate the influence of tobacco and alcohol in their study.More concrete evidence is needed to determine whether
infection is simply associated with or has a causal relation with smoking and drinking among patients with laryngeal-pharyngeal cancer.Furthermore,given the lack of the temporality between laryngeal cancer and
infection,the causal relation cannot be defined by these studies.Finally,almost all these studies were case-control studies with potential recall and selection biases that potentially influenced the outcomes of the present research.
The waiting-maid now mounted Falada, and the real bride the worse horse, and so they continued their journey till at length they arrived at the palace yard. There was great rejoicing over the arrival, and the Prince sprang forward to meet them, and taking the waiting-maid for his bride, he lifted her down from her horse and led her upstairs to the royal chamber13. In the meantime the real Princess was left standing14 below in the courtyard. The old King,25 who was looking out of his window, beheld15 her in this plight16, and it struck him how sweet and gentle, even beautiful, she looked. He went at once to the royal chamber, and asked the bride who it was she had brought with her and had left thus standing in the court below.
Summary
Current studies demonstrate the existence of
in the laryngeal mucosa (Tables 2 and 3) and support a possible connection between
infection and laryngeal cancer,but this relationship is not noted in pharyngeal cancer.The etiological mechanism of
-induced laryngeal squamous cell carcinoma is unclear,and related studies are lacking.Further evaluation of the cause-effect of
infection and pharyngeal-laryngeal cancer is required.
In the south-west, a mile from Grenen, lies Old Skjagen;merchant Bronne dwelt here, and this was also to be Jurgen s homefor the future. The dwelling-house was tarred, and all the smallout-buildings had been put together from pieces of wreck. There was no fence, for indeed there was nothing to fence in except the long rows of fishes which were hung upon lines, one above the other, to dry in the wind. The entire coast was strewn with spoiled herrings, for there were so many of these fish that a net was scarcely thrown into the sea before it was filled. They were caught by carloads, and many of them were either thrown back into the sea or left to lie on the beach.
ESOPHAGEAL CANCER
Epidemiology of esophageal cancer
Esophageal cancer constitutes 5.3% of all global cancer deaths and affects greater than 570000 people worldwide.However,the incidence rate varies across regions and populations[35].Esophageal cancer can be categorized into two main subtypes:Esophageal squamous-cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC).In past years,ESCC accounted for 70% of all esophageal cancer cases,and EAC has observed a significant and sustained rise in Western industrialized countries[36].ESCC exhibits severe geographic distribution differences:The incidence rate is highest in Eastern to Central Asia followed by the Indian Ocean coast and can exhibit greater than tenfold differences among countries.On the other hand,the prevalence of EAC increased in several regions,such as North America and Europe[36,37].In addition,the global incidence of esophageal cancer in men is 70%,and the cumulative risk from birth to 74 years of age is also higher in men compared with women (1.15%
0.43%,respectively)[35].Regarding subtypes,men have a higher risk for developing both ESCC and EAC than women with three-to fourfold and seven-to tenfold differences for each type[38].The incidence of esophageal carcinoma increases with age,peaks in the seventh and eighth decades of life,and is rare in younger people[37].
Pathological and etiological differences in esophageal cancer
ESCC and EAC exhibit very different biological presentations.ESCC is primarily found in the middle third of the esophagus,whereas EAC is located more often in the distal third of the esophagus[39].Several dietary habits are related to both types of esophageal cancer.For example,a high intake of red meats,fats,and processed foods is linked to an increased risk,whereas a high intake of fiber,fresh fruits,and vegetables is associated with a lower risk[37].Other major risk factors differ in these two types of esophageal cancer.ESCC is three to five times as likely to occur in people who consume alcohol (three or more drinks daily)[37].Smoking or betel quid chewing also increase the risk of ESCC.In addition,the combination of alcohol intake and smoking has a synergistic effect in increasing ESCC risk[37,40].The absolute risk of EAC developing in an individual 50 years of age or older is approximately 0.04% per year,and that risk is approximately twice as high among current smokers as it is among people who have never smoked[37,41].The first risk factor reported for EAC was gastroesophageal reflux disease (GERD),which was identified in the 1990s[42].Several significant associations between two of the common GERD symptoms,
heartburn sensation and acid regurgitation,and the risk of EAC have been demon-strated by several studies.When heartburn symptoms presented for at least 30 years,the risk of EAC was 6.2-fold greater than that in individuals without heartburn[43].The increasing prevalence of GERD combined with the declining prevalence of
infection has been hypothesized to be related to the increasing incidence of EAC.
Role of H.pylori in esophageal cancer
Rokkas
[44] showed no consistent association between
infection and ESCC.Unlike ESCC,several studies have found that
infection is prevalent and leads to a reduced risk of EAC (OR:0.50-0.57)[44-46].Xie
[47] showed that the risk of adenocarcinoma decreased by 41% among persons with
infection.Since the middle of the twentieth century,the prevalence of
infection has decreased in Western populations,and an increasing incidence of EAC has occurred.Scientists have proposed that the elevated incidence of EAC might result from the decreased
infection rate in these populations[48,49].The possible mechanism of this bacterial infection effect might involve
infection-induced host atrophic gastritis formation followed by reduced volume and acidity of gastric juice.Finally,this situation could counteract GERD and thereby reduce the risk of EAC[50].Further meta-analysis studies also supported the notion of a decreased risk of EAC up to 40%-60% and an OR of 0.56 for
infection (95%CI:0.46-0.68,
<0.05)[46,47].
As the most frequent location for extranodal lymphoma,the gastrointestinal tract represents 5%-20% of all cases[81].However,primary gastrointestinal lymphoma is very rare.It only constitutes approximately 1%-4% of all gastrointestinal cancers.It is slightly male predominant with a men-women ratio of 3:2.Lymphoma incidence exhibits a double peak:One in patients younger than 10 years old and another in those with a mean age of 53 years[82].
Role of host genetic effects in esophageal cancer
Past studies have shown that esophageal cancer might not be associated with family history.However,in China,studies have demonstrated an approximately two-fold increased risk of ESCC in patients with first-degree relatives who have ESCC[51,52].This situation might be explained by family members sharing some habitual factors,such as diet,obesity,alcohol and smoking.Several genetic disorders have been thought to be related to ESCC.For example,the concentrations of acetaldehyde after alcohol consumption are higher in persons with particular variants in the acetaldehyde dehydrogenase gene and the aldehyde dehydrogenase 2 family gene.If patients had these polymorphic variants,the risk of ESCC was increased up to 43-to 73-fold[53].In all ESCC individuals,83% had TP53 mutations,76% exhibited EGFR overexpression,46% harbored CCND1 mutations and 24% had CDK4/CDK6 mutations[40].In EAC patients,19% exhibited CCNE1 amplification,and 17% harbored cyclin E and MGST1 mutations[40].These genetic studies might help us to detect esophageal cancer in earlier stages[54].In addition to the above description,there are several known risk factors related to esophageal cancer.Excess intake of processed foods,hot foods and red meat was associated with an increased risk of both ESCC and EAC,and an increased intake of fresh fruits,vegetables and fiber was associated with a lower risk[37].Obesity and increased body mass index (BMI) were also thought to be associated with EAC.In particular,if the increase in BMI began in childhood or adolescence,the EAC risk seemed to be stronger than if the increase in BMI began in adulthood[55].
Summary
Unlike other gastrointestinal tract malignancy diseases,
infection might indicate a decreased risk of EAC and be unrelated to ESCC.The OR of EAC in
-infected participants was 0.56 (95%CI:0.46-0.68,
<0.05) (Tables 2 and 4).
infection might increase atrophic gastritis in the host and decrease gastric acid formation,leading to a decrease in GERD and the probability of EAC.To prevent esophageal cancer,the elimination of smoking and alcohol and very hot food or drink consumption and the practice of healthy dietary habits are beneficial.
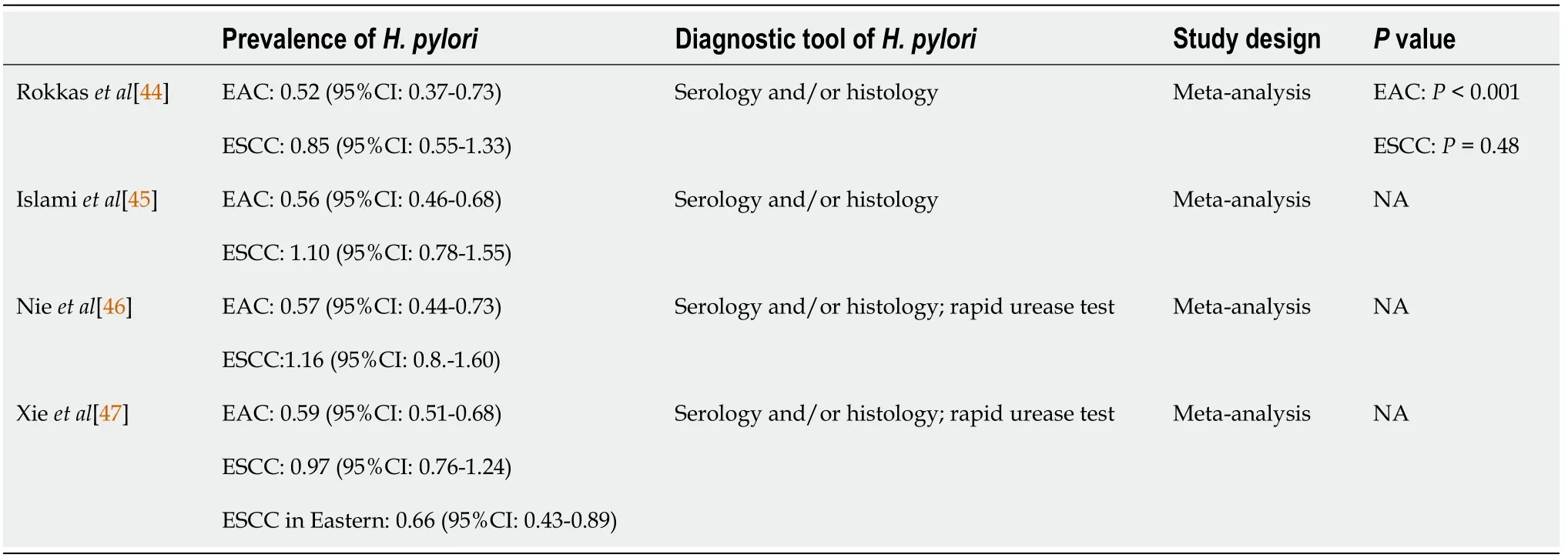
GASTRIC ADENOCARCINOMA
Epidemiology and etiology of GC
Pharyngeal-laryngeal cancer is a common malignancy of the upper aerodigestive tract.The prevalence is greater in people over the age of 60 and in males (5.8 cases per 100000 in males
1.2 per 100000 in females)[20].Pharyngeal-laryngeal cancer comprises 2%-3% of the malignancies of the whole body and constitutes 25% of head and neck cancers[21].In addition,racial differences were noticed with a younger age and a higher incidence and mortality in African Americans than in Caucasians[22,23].Moreover,the younger (<40 years old) the patients were diagnosed,the more aggressive and the poorer the survival rate[24].Major risk factors for pharyngeallaryngeal cancer include cigarette smoking and alcohol consumption.A study examining the effect of alcohol consumption and smoking in laryngeal cancer reported that the adjusted odds ratios for nonsmoking heavy drinkers (defined as>8 drinks per day) and for nondrinking smokers were 2.46 and 9.38,respectively[25].Microbes,viruses,occupational exposures,gastroesophageal reflux,and genetic inheritance,for example,were also linked to malignancy[26].
Burduk
[33] showed a correlation of a high incidence of positivity for the
cytotoxin-associated gene A (
) gene in laryngeal cancer tissue (46.7% to 49.3%) and a reduced survival rate.However,several studies failed to demonstrate the direct correlation between
and laryngeal cancer.A study found a significantly higher frequency of
colonization at the antrum compared with the gastric body in patients with laryngeal cancer.It was hypothesized that
in the antrum reduces gastric acid when colonizing the body and increases by G cell hyperplasia,thus leading to laryngeal cancer through gastric reflux[34].
has been identified in some laryngeal diseases.Given the lack of reliable research,the role of
in the larynx remains unclear.
Role of H.pylori in GC
In 1982,Warren and Marshal[60] found a connection between
and gastric ulcer disease,and since then,this bacterium has become a topic of study in the gastroenterology field.Twelve years later,the International Agency for Research on Cancer recognized
as a class I carcinogen[61].For general microorganisms,the stomach environment is not suitable for survival because the gastric acid and pH level is less than 0.3-2.9[62].However,with the assistance of urease-derived ammonia,
can buffer cytosolic,periplasmic and surface acidity in such an extreme environment of the stomach[63].This environment might induce
to become the predominant microorganism in the stomach.In addition,the gastric transit time was greater than 2-4 h (Table 2),giving
more chances to attach to the stomach.When
strains carry the cag pathogenicity island (
PAI),the risk of peptic ulcer disease or GC increases.With a size of 40 kb,
PAI contains 30 genes,including
[64].A previous study showed that
-infected people had an approximately sixfold increased risk of developing non-cardia GCs (OR:5.9;95%CI:3.4-10.3)compared with uninfected individuals[65].Furthermore,compared to infection with
-negative strains,a 1.64-fold (95%CI:1.21-2.24) increased risk of GC was found for
-positive strains[66].In gastric epithelial cells,a cell scattering effect caused by cytoskeletal modifications and proinflammatory responses triggered by the transcription factor NF-κB were observed when a functional
PAI was present in
[67,68].Activation of growth factor receptors,cell proliferation,inhibition of apoptosis,invasion and angiogenesis occurred through
A[69].
The connection between
infection and GC was most significant in whole gastrointestinal tract cancer.
infection increases GC incidence,but GC incidence is decreased after
eradication.Lee
[70] demonstrated an association of
infection eradication with a reduced incidence of GC in a metaanalysis study.After adjustment for baseline GC incidence,the pooled incidence rate for individuals receiving
eradication treatment was 0.53 (95%CI:0.44-0.64).Recently,the long-term benefits of eradication were confirmed by Chiang
[71],revealing a significant reduction in the occurrence of GC by 53% for a high-risk Taiwanese population.From 2004 to 2018,a mass eradication program was conducted in patients older than 30 years old on the Matsu Islands,where
infection was prevalent.After
eradication,the infection rates declined from 64% to 15%.GC incidence and mortality after the chemoprevention period were reduced to 53%(95%CI:0.3-0.69) and 25% (95%CI:0.14-0.51),respectively.The 2020 Taipei global consensus supported that “eradication therapy should be offered to all individuals infected with
” and suggested that screen-and-treat is a cost-effective strategy for young adults in GC high incidence areas at the general population level[72].
Role of host genetic effects in GC
In addition to
infection,dietary habits,lifestyle,family history and occupational exposure are also risk factors for GC.Fresh fruits and vegetables are protective against GC.Compared to individuals who intake less than one serving fruit and vegetable
day,participants who ate 2-5 servings had a hazard ratio (HR) of 0.56(95%CI:0.34-0.93)[73].Some scientists have suggested that this might be related to an increase in vitamin C in fresh fruits and vegetables[74].On the other hand,pickled vegetables,dried fish,and salted fish were associated with an increased incidence of GC[75].High dietary salt intake was also associated with an increased risk of GC when salt intake was more than 10 g per day[76].Regarding lifestyle,alcohol intake and smoking were thought to increase GC incidence.Duell
[77] found that modest alcohol intake of greater 60 grams per day would increase the risk of GC to 1.65(95%CI:1.06-2.58).The meta-analysis conducted by Ladeiras-Lopes
[78] included 42 studies from Asia,Europe and the United States and reported a relative risk of 1.53 for smokers (1.62 males and 1.2 females).Smoking not only increased GC risk but also affected GC recurrence and survival.As an independent risk factor,smokers had a significantly worse 5-year disease-free survival (HR:1.46,
=0.007) and overall survival (HR:1.48,
=0.003) than nonsmokers[79].The GC risk was increased two-to threefold in first-degree relatives of patients with this disease.This finding might be due to the familial clustering trend of
infection[80].Occupational exposures to dust and heat,such as those experienced by chefs,wood processing plant operators,food processing and related trade workers,and machine operators,was linked to a significantly raising risk of diffuse GC[67].
Summary
In the whole gastrointestinal tract,GC was most related to
infection,especially in non-cardia GCs.The OR of GC in
-infected participants was 5.9(95%CI:3.4-10.3,
<0.05) (Tables 2 and 5).The host organ environment and pathogen characteristics might explain this result.The very low pH level in the stomach allows
to predominate in this niche,and adequate gastric transit time provides this bacterium with a greater chance of colonization in the stomach.
strains with a functional
PAI further increased the risk for GC by 1.64-fold.Based on the 2020 Taipei global consensus,mass screening and eradication of
are necessary to prevent GC in high-risk populations.
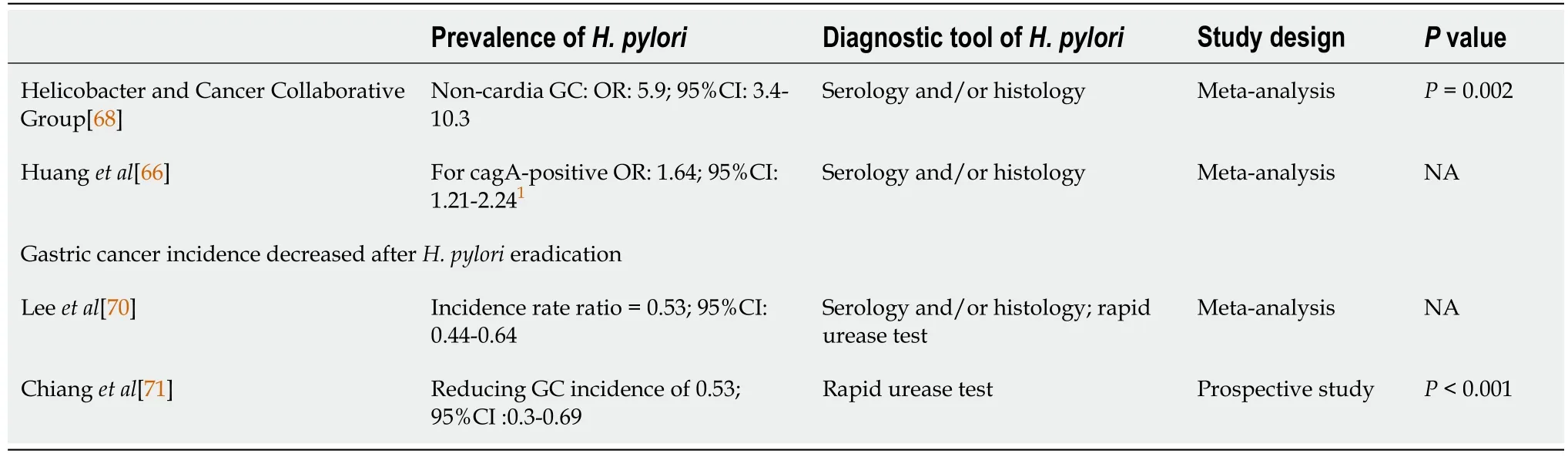
GASTROINTESTINAL TRACT LYMPHOMA
Epidemiology of gastrointestinal tract lymphoma
They declared that the pedlar, in fear of his life, had complained to the king; and that they had been sent to bring the lawless person who had said these things before the king himself
The prevalence of lymphoma among different gastrointestinal locations is highest for the stomach (60%-75%) followed by the small intestine,ileocecal region and rectum[83].With an elevated incidence worldwide,non-Hodgkin’s lymphomas (NHLs) are the most common primary gastric lymphomas,accounting for 5% of gastric malignancies[84].Primary small intestinal lymphoma occurrence is comparatively rare,constituting 19%-38% of small intestine cancers[85],20%-30% of primary gut lymphomas[86],and 4%-12% of all NHLs[87].The most frequent location of small intestine lymphoma involvement is the ileum (60%-65%) followed by the jejunum(20%-25%) and duodenum (6%-8%)[88].
So the tailor gave him some more pebbles, and the bear bit and gnawed10 away as hard as he could, but I need hardly say that he did not succeed in cracking one of them
He brought the stranger into his hall, and after they had supped went again to speak to Morgiana in the kitchen, while the Captain went into the yard under pretence26 of seeing after his mules, but really to tell his men what to do
Colorectal lymphoma constitutes 6%-12% of all gastrointestinal lymphomas.Simply contributing 0.2% of all cancers,it is very rare for primary colorectal lymphoma[89].The most common sites of tumor growth are the cecum (71.5%),rectum (16.9%),and ascending colon (6.2%),whereas the sigmoid colon is rarely involved[90].Primarily occurring from the fourth to the seventh decades of life,primary colorectal lymphomas are diagnosed at an average age of 50 years.Males are affected approximately twofold more frequently than females[91].
Pathological differences in gastrointestinal tract lymphoma
Histopathologically,approximately 90% of primary gastrointestinal lymphomas are of the B cell lineage.Among them,over 90% are mucosa-associated lymphoid tissue(MALT) lymphoma and diffuse large B-cell lymphoma (DLBCL).Notably,MALT lymphoma constitutes half of all primary lymphomas with gastric involvement[92].
Primary small intestine lymphomas that are more heterogeneous than those in the stomach include MALT lymphoma,DLBCL,enteropathy-associated T-cell lymphoma,mantle cell lymphoma (MCL),follicular lymphoma and immunoproliferative lymphoma[93].
Primary colorectal lymphomas include MALT-related low-grade B-cell lymphoma,MCL,and peripheral T-cell lymphoma.Manifesting as multiple polyps,MCL is aggressive.In contrast,low-grade B-cell lymphoma derived from MALT is indolent and occasionally appears as multiple polyps.Colonic peripheral T-cell lymphoma expresses as either a diffuse or a focal segmental lesion with extensive mucosal ulceration[94].
Will you be off at once? So he was frightened and went out; but he felt quite faint, and trembled and shook, and his knees and legs began to give way under him
Role of H.pylori in gastrointestinal tract lymphoma
A previous large population-based study,in which the seroprevalence of
was higher in patients with gastric lymphoma than in matched controls,confirmed the relationship between
-related chronic gastritis and MALT lymphoma[95].Gastric MALT lymphoma is highly correlated with
in 72%-98% of low-grade cases[96].In a retrospective study conducted by Parsonnet
[95],
seropositivity preceded the diagnosis of gastric NHL for years (OR:6.3;95%CI:2.0-19.9).MALT lymphoma was positively correlated with
infection (OR:1.96;95%CI:1.0-3.9)[97].The regression of low-grade gastric MALT lymphoma after the eradication of
has been described by some recent studies[98]
Epidemiological and experimental data support the hypothesis that
can serve as an antigenic stimulus supporting the growth of gastric lymphoma.Polymorphisms in host genes regulating the inflammatory response and antioxidative mechanisms in gastric MALT lymphoma patients suggest a correlation with the capacity to neutralize free radicals,and individual variations in the inflammatory response to
have been observed in recent research[99].Expression of the CagA protein by
strains induced severe gastritis or even peptic ulcerations.The hypothesis that CagA+
strains are linked to the development of gastric MALT lymphomas is observed in nearly all cases of patients in whom anti-CagA antibodies are present at a higher rate compared with inactive gastritis cases[100].
Parsonnet
[95] failed to demonstrate a correlation between non-gastric NHL and prior
infection (OR:1.2;95%CI:0.5-3.0).Several cases of colorectal MALT lymphoma that disappeared completely after
eradication were presented in 1998[101].Unlike gastric MALT lymphomas,which can be successfully treated by
eradication alone,colorectal MALT lymphomas,which have different relationships with
infection,act and are viewed as a distinct clinical entity.However,antibiotic treatment against
is effective for colonic MALT lymphoma,and this treatment even influences
-negative patients[102].
Role of the host effect in gastrointestinal tract lymphoma
Sixty-five percent of gastric MALT lymphomas present with chromosomal translocations,including the t(14;18)(q32;q21) translocation,which causes deregulation of MALT1;the t(11;18)(q21;q21) translocation,which causes the formation of the chimeric fusion gene AP12-MALT1;and the t(1;14)(p22;q32) translocation,which causes deregulation of BCL10.Through the regulation of different genes,these translocations are involved in immunity,inflammation and apoptosis[103].
Polymorphisms of specific cytokines have been researched in the context of MALT lymphoma.Upregulation of IL-1 production is typically noted in the presence of
[104].High IL-1 levels favor a proinflammatory response.In combination with the inhibition of gastric acid,extensive
colonization is facilitated,and MALT growth is promoted[99].
Acting on the signaling pathway,tumor necrosis factor (TNF) and its receptors greatly influence the immune response.TNF may accelerate the growth of lymphoid cells
,and high concentrations of TNF were detected in patients with malignant lymphoma[105].
When Charles went to Switzerland for a ski vacation, Diana missed him terribly. He called her after a day or two, and told Diana he had something important to ask her.
A well-known oncogene,Bcl-6,which is located on the long arm of chromosome 3,is found in most extranodal high-grade lymphomas.Its overexpression was also reported in gastric DLBCL[106].
Summary
Gastrointestinal lymphoma is a relatively rare disease with a diverse clinical presentation.The epidemiology and histopathologic subtypes as well as their relationship with
infection,are highlighted in this review.For gastric MALT lymphoma,a positive association with
infection was found (OR:1.96;95%CI:1.0-3.9,
<0.05) (Tables 2 and 6).Other non-gastric MALT lymphomas did not show this association.
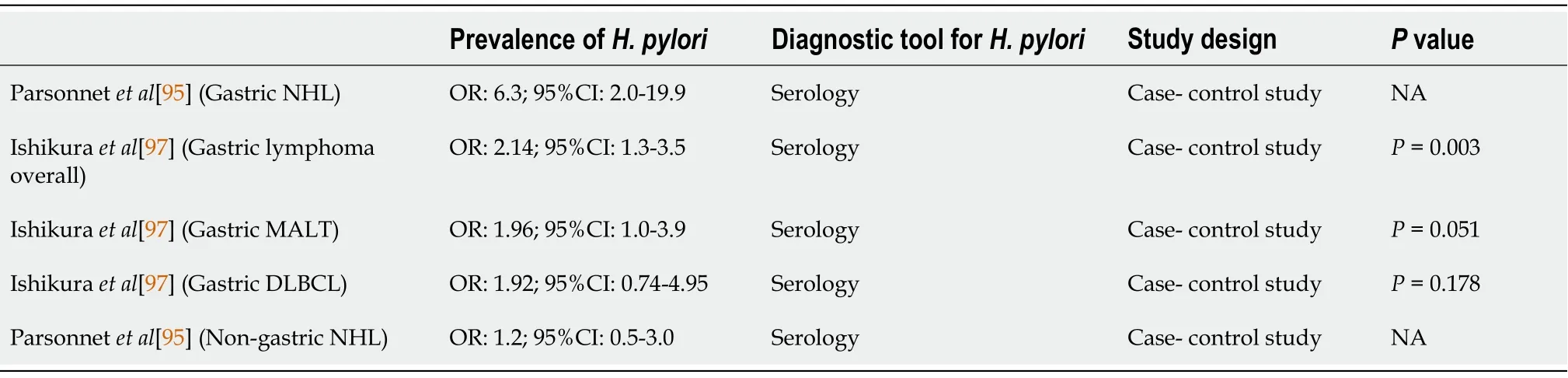
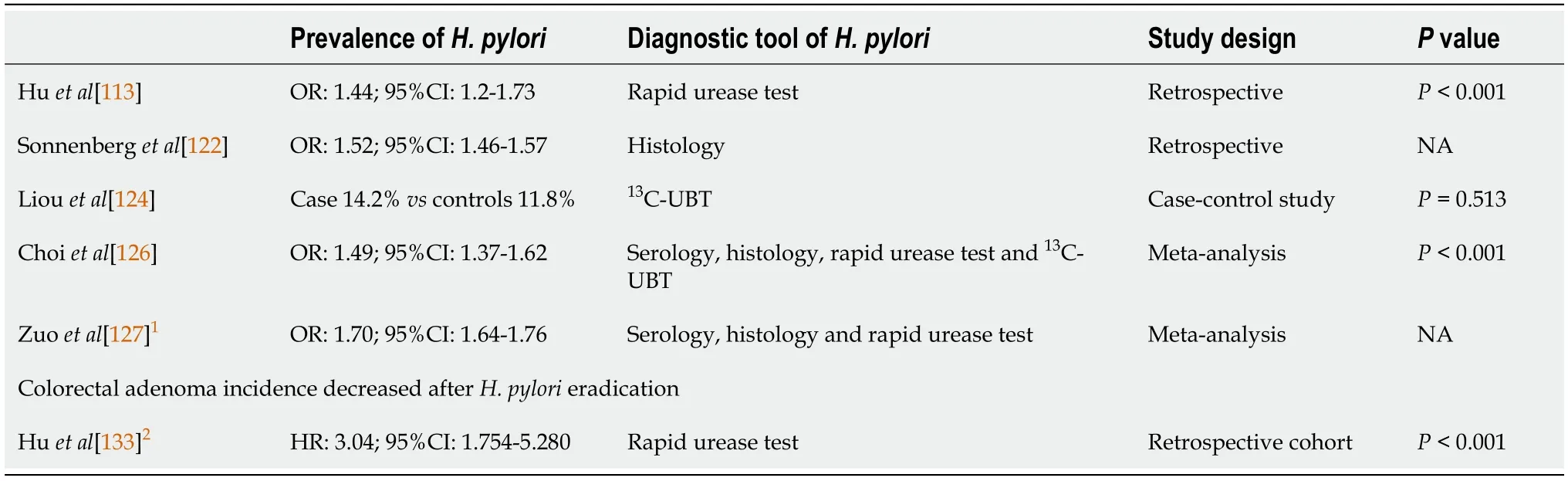
CRC
Epidemiology of CRC
CRC is the third most commonly diagnosed cancer in males and the second most commonly diagnosed cancer in females worldwide[107].In the United States,CRC ranks as the second leading cause of cancer mortality in the population.This trend was similar in Europe,Australia and New Zealand,and these countries showed higher CRC incidence rates[108].Japan,Thailand,Saudi Arabia and Iran have suffered rapid increases in CRC incidence over the past 30 years[109-111].However,the agestandardized incidence rates vary in different countries.The country with the highest incidence rate was hungary,which had 51.2 cases per 100000 persons per year,and the country with the lowest incidence rate was Gambia with 1.1 cases per 100000 persons per year.The cause of this variation might be due to several factors,such as lifestyle,genetics,economic status (for example,meat consumption) and life expectancy (for example,some underdeveloped countries had lower CRC incidence rates because fewer people reach ages over 65 years,when most CRC is diagnosed)[107,112].It is worth noting that some countries had a low CRC risk regardless of a high prevalence of
.This finding challenges the connection between
and CRC development.
This result might be explained by the fact that CRC has multiple contributing factors and
infection is one of them.For example,together with hyperglycemia,
infection has a synergistic effect on the risk of colon adenoma[113].Areas with a higher prevalence of
infection but lower incidence of CRC,including Asia,some eastern European countries,and specific countries in South America,exhibit a lower diabetes prevalence[114].This finding indicates that if the DM prevalence increases,the CRC prevalence might be elevated,which leads to areas with a higher prevalence of
infection but lower CRC incidence rates.
Pathological differences in CRC
There are three major pathologic pathways of CRC:The adenoma-carcinoma sequence,the serrated pathway and the inflammatory pathway.An estimated 85%-90% of sporadic CRC cases are derived from the adenoma-carcinoma sequence.In this pathway,several stepwise accumulations of genetic and epigenetic alterations drive the transformation of normal colon mucosal cells into an adenoma.First,the inactivated tumor suppressor gene
is regarded as the gatekeeper against colorectal neoplasms.Second,
,an oncogene mutation,facilitates adenoma growth.Then,inactivation of the tumor suppressor gene (
) promotes CRC progression[115,116].Approximately 10%-15% of sporadic CRC is caused by the serrated pathway.This pathway includes several gene mutations.Oncogene
mutations induce uncontrolled cell proliferation and contribute to the formation of hyperplastic polyps through constitutive activation of the MAPK pathway[117].Then,hypermethylation at repetitive CG dinucleotides CpG island methylator phenotype(CIMP) results in mutations in the promoter regions of tumor suppressor genes.CIMP presents cell progression to sessile serrated adenoma and CRC.Approximately 75% of sessile serrated adenomas and 90% of serrated adenocarcinomas had CIMP-positive presentations[118,119].Less than 2% of all CRC is caused by the inflammatory pathway.In this path-way,normal colon mucosal cells progress from indefinite dysplasia to low-grade dysplasia,high-grade dysplasia and cancer due to chronic inflammation[107].
Role of H.pylori in CRC
Since the 1990s,the connection between
and colorectal neoplasm formation has been widely discussed by scientists.Most reports demonstrated that
was linked to both benign and malignant colon lesions.For instance,
contributes to an elevated risk of 1.3-to 1.97-fold for colon adenoma with or without high-grade dysplasia[113,120-123].Some scientists did not agree because their data revealed an insignificant increase in colon adenoma in combination with
infection[124,125].Nonetheless,two recent meta-analysis studies uncovered a significant and positive correlation between
infection and the risk of colorectal adenoma (OR:1.49,95%CI:1.37-1.62)[126] and CRC (OR:1.70;95%CI:1.64-1.76,
=97%)[127].The potential mechanisms for
-induced colorectal neoplasms might include direct and/or indirect effects.However,a few studies have shown positive
PCR histology in colon tumors and found
in 22%-27% of colorectal polyps or cancers[128,129].Recent studies favored the associations between CRC and bloodstream infections caused by
(
),
(
) and
(
)[130].Thus,
and
could have direct effects on the formation of colon neoplasms or cancer.
So they dug a hole, and then the little hare said, The next thing is to make a fire in the hole, and they set to work to collect wood, and lit quite a large fire
might affect colorectal tumors through indirect effects.In the whole gastrointestinal tract,the colonic transit time is the longest[131] (Table 2).The long transit time offers more opportunities for
to alter the colonization of the colon,in which other bacteria might promote the development of neoplasms.Additionally,
enhances the release of gastrin,which contributes to colorectal carcinogenesis,possibly through its mitogen activity.
also appears to be associated with metabolic diseases with established connections with CRC.Finally,systemic inflammatory responses triggered by
-induced chronic inflammation of the gastric epithelium may increase the risk of CRC[132].Although the possible mechanism of
induced CRC was indirect,our previous study demonstrated a reduced risk of colorectal adenoma after successful eradication therapy[133].This result implies that
is related to colon neoplasm formation by being a “biomarker” or “indicator organism”,reflecting exposure to immune-stimulating carcinogenic bacteria or antigens.
” And while she went on combing little Gerda’s hair, she thought less and less about her adopted brother Kay, for the old woman could conjure9, although she was not a wicked witch; she conjured10 only a little for her own amusement, and now, because she wanted to keep Gerda
Role of the host effect in CRC
In addition to
,several host factors and other environmental factors potentially contribute to the pathogenesis of CRC.Risk factors for colorectal neoplasms included age 60 years or older,male sex,obesity,diet,dyslipidemia,impaired glucose tolerance,a family history of CRC,alcohol intake,tobacco use,and sedentary lifestyle[134-136].Most of these risk factors were associated with metabolic syndrome.Compared with healthy individuals,patients with hyperglycemia have a higher prevalence of colonic neoplasms (26.6%
16.5%,
<0.001)[137].Waist circumference,one of the components of metabolic syndrome,was an independent risk factor for colorectal adenoma,and diabetes mellitus type 2 had an OR of 1.38 for CRC[138].Compared to non-or occasional drinkers,people who consume four more drinks per day have a 72% increased risk of developing CRC.Cigarette smoking increased the risk of CRC approximately two-to threefold compared with nonsmokers[139].
Summary
infection might indicate an increased risk of CRC.The OR of CRC in
infected participants was 1.70 (95%CI:1.64-1.76,
<0.05) (Tables 2 and 7)[127].Although
infection might have an indirect effect on the formation of CRC,the presence or absence of this bacterium could remind clinicians of the possibility of CRC.
eradication therapy benefits both gastric malignancies and colorectal neoplasms by reducing their occurrence.
CONCLUSION
Given that
infection is an important infectious disease worldwide and affects human health through correlation with several diseases,such as gastric ulcers,GC and gastric MALT lymphoma,further realization of the effects of this bacterium in other gastrointestinal tract diseases is necessary.
infection induces chronic inflammatory changes in the human body and then increases GC,gastric MALT lymphoma and colorectal adenoma formation.In addition,an inverse relationship between
infection and EAC formation was observed due to atrophic gastritis and decreased gastric acid formation.From a microorganism viewpoint,the host gastroenterological microenvironment and motility status might play an important role in deciding which bacteria could colonize organs and subsequently induce chronic inflammatory and malignant changes in host organs.Further evaluation of human and bacterial interactions might allow us to better understand disease treatment.
1 Ferlay J,Ervik M,Lam F,et al Global Cancer Observatory:Cancer Today.Lyon,France:International Agency for Research on Cancer.[cited 28 January 2021].Available from:https://gco.iarc.fr/today
2 de Martel C,Georges D,Bray F,Ferlay J,Clifford GM.Global burden of cancer attributable to infections in 2018:a worldwide incidence analysis.
2020;8:e180-e190 [PMID:31862245 DOI:10.1016/S2214-109X(19)30488-7]
3 Mandeville KL,Krabshuis J,Ladep NG,Mulder CJ,Quigley EM,Khan SA.Gastroenterology in developing countries:issues and advances.
2009;15:2839-2854 [PMID:19533805 DOI:10.3748/wjg.15.2839]
4 Go MF.Review article:natural history and epidemiology of Helicobacter pylori infection.
2002;16 Suppl 1:3-15 [PMID:11849122 DOI:10.1046/j.1365-2036.2002.0160s1003.x]
5 Hooi JKY,Lai WY,Ng WK,Suen MMY,Underwood FE,Tanyingoh D,Malfertheiner P,Graham DY,Wong VWS,Wu JCY,Chan FKL,Sung JJY,Kaplan GG,Ng SC.Global Prevalence of Helicobacter pylori Infection:Systematic Review and Meta-Analysis.
2017;153:420-429 [PMID:28456631 DOI:10.1053/j.gastro.2017.04.022]
6 Infection with Helicobacter pylori.
1994;61:177-240[PMID:7715070]
7 Tobacco habits other than smoking;betel-quid and areca-nut chewing;and some related nitrosamines.IARC Working Group.Lyon,23-30 October 1984.
1985;37:1-268 [PMID:3866741]
8 MEHTA FS,SANJANA MK,BARRETTO MA.Relation of betel leaf chewing to periodontal disease.
1955;50:531-536 [PMID:14366931 DOI:10.14219/jada.archive.1955.0098]
9 Agni NA,Prasad G,Borle RM,Shukla S,Grover S,Korde S.Assessment of perineural infiltration and spread of oral squamous cell carcinoma:a clinicohistopathologic study.
2010;47:199-205 [PMID:20448387 DOI:10.4103/0019-509X.63024]
10 Müller S,Pan Y,Li R,Chi AC.Changing trends in oral squamous cell carcinoma with particular reference to young patients:1971-2006.The Emory University experience.
2008;2:60-66 [PMID:20614324 DOI:10.1007/s12105-008-0054-5]
11 Mohtasham N,Babakoohi S,Salehinejad J,Montaser-Kouhsari L,Shakeri MT,Shojaee S,Sistani NS,Firooz A.Mast cell density and angiogenesis in oral dysplastic epithelium and low-and highgrade oral squamous cell carcinoma.
2010;68:300-304 [PMID:20586672 DOI:10.3109/00016357.2010.494622]
12 van der Waal R,van der Waal I.Oral non-squamous malignant tumors;diagnosis and treatment.
2007;12:E486-E491 [PMID:17978771]
13 Fernando N,Jayakumar G,Perera N,Amarasingha I,Meedin F,Holton J.Presence of Helicobacter pylori in betel chewers and non betel chewers with and without oral cancers.
2009;9:23 [PMID:19772630 DOI:10.1186/1472-6831-9-23]
14 Grandis JR,Perez-Perez GI,Yu VL,Johnson JT,Blaser MJ.Lack of serologic evidence for Helicobacter pylori infection in head and neck cancer.
1997;19:216-218 [PMID:9142522 DOI:10.1002/(sici)1097-0347(199705)19:3<216::aid-hed9>3.0.co;2-5]
15 Dayama A,Srivastava V,Shukla M,Singh R,Pandey M.Helicobacter pylori and oral cancer:possible association in a preliminary case control study.
2011;12:1333-1336 [PMID:21875292]
16 Gupta AA,Kheur S,Raj AT,Mahajan P.Association of Helicobacter pylori with oral potentially malignant disorders and oral squamous cell carcinoma-a systematic review and meta-analysis.
2020;24:13-23 [PMID:31707627 DOI:10.1007/s00784-019-03125-2]
17 Meng X,Wang Q,He C,Chen M,Liu J,Liu W,Yuan Y.An inverse association of Helicobacter pylori infection with oral squamous cell carcinoma.
2016;45:17-22 [PMID:25899621 DOI:10.1111/jop.12324]
18 Pandey S,Follin-Arbelet B,Pun CB,Gautam DK,Johannessen AC,Petersen FC,Costea DE,Sapkota D.Helicobacter pylori was not detected in oral squamous cell carcinomas from cohorts of Norwegian and Nepalese patients.
2020;10:8737 [PMID:32457404 DOI:10.1038/s41598-020-65694-7]
19 Waerhaug J.Prevalence of periodontal disease in Ceylon.Association with age,sex,oral hygiene,socio-economic factors,vitamin deficiencies,malnutrition,betel and tobacco consumption and ethnic group.Final report.
1967;25:205-231 [PMID:5233925 DOI:10.3109/00016356709028749]
20 Baselga J.Why the epidermal growth factor receptor?
2002;7 Suppl 4:2-8 [PMID:12202782 DOI:10.1634/theoncologist.7-suppl_4-2]
21 Koufman JA,Burke AJ.The etiology and pathogenesis of laryngeal carcinoma.
1997;30:1-19 [PMID:8995133]
22 Goodwin WJ,Thomas GR,Parker DF,Joseph D,Levis S,Franzmann E,Anello C,Hu JJ.Unequal burden of head and neck cancer in the United States.
2008;30:358-371 [PMID:17972309 DOI:10.1002/hed.20710]
23 Shin JY,Truong MT.Racial disparities in laryngeal cancer treatment and outcome:A populationbased analysis of 24,069 patients.
2015;125:1667-1674 [PMID:25694265 DOI:10.1002/lary.25212]
24 Li R,Yu S,Zhu W,Wang S,Yan L.Studying the impact of young age on prognosis and treatment in laryngeal squamous cell carcinomas using the SEER database.
2019;7:e7368 [PMID:31380154 DOI:10.7717/peerj.7368]
25 Bosetti C,Gallus S,Franceschi S,Levi F,Bertuzzi M,Negri E,Talamini R,La Vecchia C.Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers.
2002;87:516-518 [PMID:12189548 DOI:10.1038/sj.bjc.6600469]
26 Tutar H,Erdamar H,K?yba?io?lu A,Din? AE,Ceylan A,Uslu S.Can bile acids be an etiological factor for laryngeal carcinoma?
2011;73:156-161 [PMID:21508656 DOI:10.1159/000327521]
27 Jemal A,Siegel R,Ward E,Hao Y,Xu J,Murray T,Thun MJ.Cancer statistics,2008.
2008;58:71-96 [PMID:18287387 DOI:10.3322/CA.2007.0010]
28 Sudhoff H,Rajagopal S,Baguley DM,Ebmeyer J,Schmelzer A,Schreiber S,Moffat DA.A critical evaluation of the evidence on a causal relationship between Helicobacter pylori and otitis media with effusion.
2008;122:905-911 [PMID:18036278 DOI:10.1017/S0022215107000989]
29 Ozdek A,Cirak MY,Samim E,Bayiz U,Safak MA,Turet S.A possible role of Helicobacter pylori in chronic rhinosinusitis:a preliminary report.
2003;113:679-682 [PMID:12671428 DOI:10.1097/00005537-200304000-00018]
30 Zhou J,Zhang D,Yang Y,Zhou L,Tao L.Association between helicobacter pylori infection and carcinoma of the larynx or pharynx.
2016;38 Suppl 1:E2291-E2296 [PMID:26316145 DOI:10.1002/hed.24214]
31 Siupsinskiene N,Jurgutaviciute V,Katutiene I,Janciauskas D,Vaitkus S,Adamonis K.Helicobacter pylori infection in laryngeal diseases.
2013;270:2283-2288 [PMID:23572292 DOI:10.1007/s00405-013-2475-3]
32 Zhuo XL,Wang Y,Zhuo WL,Zhang XY.Possible association of Helicobacter pylori infection with laryngeal cancer risk:an evidence-based meta-analysis.
2008;39:625-628 [PMID:18662596 DOI:10.1016/j.arcmed.2008.04.008]
33 Burduk PK.Association between infection of virulence cagA gene Helicobacter pylori and laryngeal squamous cell carcinoma.
2013;19:584-591 [PMID:23860397 DOI:10.12659/MSM.889011]
34 Pirzadeh A,Doustmohammadian N,Khoshbaten M,Doustmohammadion S.Is there any association between Helicobacter Pylori infection and laryngeal carcinoma?
2011;12:897-900 [PMID:21790222]
35 Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
2018;68:394-424 [PMID:30207593 DOI:10.3322/caac.21492]
36 Arnold M,Soerjomataram I,Ferlay J,Forman D.Global incidence of oesophageal cancer by histological subtype in 2012.
2015;64:381-387 [PMID:25320104 DOI:10.1136/gutjnl-2014-308124]
37 Rustgi AK,El-Serag HB.Esophageal carcinoma.
2014;371:2499-2509 [PMID:25539106 DOI:10.1056/NEJMra1314530]
38 Uhlenhopp DJ,Then EO,Sunkara T,Gaduputi V.Epidemiology of esophageal cancer:update in global trends,etiology and risk factors.
2020;13:1010-1021 [PMID:32965635 DOI:10.1007/s12328-020-01237-x]
39 Pickens A,Orringer MB.Geographical distribution and racial disparity in esophageal cancer.
2003;76:S1367-S1369 [PMID:14530066 DOI:10.1016/s0003-4975(03)01202-5]
40 Huang FL,Yu SJ.Esophageal cancer:Risk factors,genetic association,and treatment.
2018;41:210-215 [PMID:27986415 DOI:10.1016/j.asjsur.2016.10.005]
41 Tramacere I,La Vecchia C,Negri E.Tobacco smoking and esophageal and gastric cardia adenocarcinoma:a meta-analysis.
2011;22:344-349 [PMID:21330928 DOI:10.1097/EDE.0b013e31821092cd]
42 Chow WH,Finkle WD,McLaughlin JK,Frankl H,Ziel HK,Fraumeni JF Jr.The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia.
1995;274:474-477 [PMID:7629956]
43 Cook MB,Corley DA,Murray LJ,Liao LM,Kamangar F,Ye W,Gammon MD,Risch HA,Casson AG,Freedman ND,Chow WH,Wu AH,Bernstein L,Nyrén O,Pandeya N,Whiteman DC,Vaughan TL.Gastroesophageal reflux in relation to adenocarcinomas of the esophagus:a pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON).
2014;9:e103508 [PMID:25075959 DOI:10.1371/journal.pone.0103508]
44 Rokkas T,Pistiolas D,Sechopoulos P,Robotis I,Margantinis G.Relationship between Helicobacter pylori infection and esophageal neoplasia:a meta-analysis.
2007;5:1413-1417,1417.e1 [PMID:17997357 DOI:10.1016/j.cgh.2007.08.010]
45 Islami F,Kamangar F.Helicobacter pylori and esophageal cancer risk:a meta-analysis.
2008;1:329-338 [PMID:19138977 DOI:10.1158/1940-6207.CAPR-08-0109]
46 Nie S,Chen T,Yang X,Huai P,Lu M.Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma:a meta-analysis.
2014;27:645-653[PMID:24635571 DOI:10.1111/dote.12194]
47 Xie FJ,Zhang YP,Zheng QQ,Jin HC,Wang FL,Chen M,Shao L,Zou DH,Yu XM,Mao WM.Helicobacter pylori infection and esophageal cancer risk:an updated meta-analysis.
2013;19:6098-6107 [PMID:24106412 DOI:10.3748/wjg.v19.i36.6098]
48 Banatvala N,Mayo K,Megraud F,Jennings R,Deeks JJ,Feldman RA.The cohort effect and Helicobacter pylori.
1993;168:219-221 [PMID:8515114 DOI:10.1093/infdis/168.1.219]
49 Rehnberg-Laiho L,Rautelin H,Koskela P,Sarna S,Pukkala E,Aromaa A,Knekt P,Kosunen TU.Decreasing prevalence of helicobacter antibodies in Finland,with reference to the decreasing incidence of gastric cancer.
2001;126:37-42 [PMID:11293681]
50 Atherton JC,Blaser MJ.Coadaptation of Helicobacter pylori and humans:ancient history,modern implications.
2009;119:2475-2487 [PMID:19729845 DOI:10.1172/JCI38605]
51 Turati F,Edefonti V,Bosetti C,Ferraroni M,Malvezzi M,Franceschi S,Talamini R,Montella M,Levi F,Dal Maso L,Serraino D,Polesel J,Negri E,Decarli A,La Vecchia C.Family history of cancer and the risk of cancer:a network of case-control studies.
2013;24:2651-2656[PMID:23884440 DOI:10.1093/annonc/mdt280]
52 Su Z,Zou GR,Mao YP,OuYang PY,Cao XL,Xie FY,Li Q.Prognostic impact of family history of cancer in Southern Chinese patients with esophageal squamous cell cancer.
2019;10:1349-1357 [PMID:31031844 DOI:10.7150/jca.26511]
53 Yokoyama T,Yokoyama A,Kato H,Tsujinaka T,Muto M,Omori T,Haneda T,Kumagai Y,Igaki H,Yokoyama M,Watanabe H,Yoshimizu H.Alcohol flushing,alcohol and aldehyde dehydrogenase genotypes,and risk for esophageal squamous cell carcinoma in Japanese men.
2003;12:1227-1233 [PMID:14652286]
54 Yokoyama T,Yokoyama A,Kumagai Y,Omori T,Kato H,Igaki H,Tsujinaka T,Muto M,Yokoyama M,Watanabe H.Health risk appraisal models for mass screening of esophageal cancer in Japanese men.
2008;17:2846-2854 [PMID:18843030 DOI:10.1158/1055-9965.EPI-08-0397]
55 Petrick JL,Kelly SP,Liao LM,Freedman ND,Graubard BI,Cook MB.Body weight trajectories and risk of oesophageal and gastric cardia adenocarcinomas:a pooled analysis of NIH-AARP and PLCO Studies.
2017;116:951-959 [PMID:28196067 DOI:10.1038/bjc.2017.29]
56 Rawla P,Barsouk A.Epidemiology of gastric cancer:global trends,risk factors and prevention.
2019;14:26-38 [PMID:30944675 DOI:10.5114/pg.2018.80001]
57 Mukaisho K,Nakayama T,Hagiwara T,Hattori T,Sugihara H.Two distinct etiologies of gastric cardia adenocarcinoma:interactions among pH,Helicobacter pylori,and bile acids.
2015;6:412 [PMID:26029176 DOI:10.3389/fmicb.2015.00412]
58 Balakrishnan M,George R,Sharma A,Graham DY.Changing Trends in Stomach Cancer Throughout the World.
2017;19:36 [PMID:28730504 DOI:10.1007/s11894-017-0575-8]
59 Mu?oz N,Franceschi S.Epidemiology of gastric cancer and perspectives for prevention.
1997;39:318-330 [PMID:9337564 DOI:10.1590/s0036-36341997000400010]
60 Warren JR,Marshall B.Unidentified curved bacilli on gastric epithelium in active chronic gastritis.
1983;1:1273-1275 [PMID:6134060]
61 M?ller H,Heseltine E,Vainio H.Working group report on schistosomes,liver flukes and Helicobacter pylori.
1995;60:587-589 [PMID:7860130 DOI:10.1002/ijc.2910600502]
62 Ayazi S,Leers JM,Oezcelik A,Abate E,Peyre CG,Hagen JA,DeMeester SR,Banki F,Lipham JC,DeMeester TR,Crookes PF.Measurement of gastric pH in ambulatory esophageal pH monitoring.
2009;23:1968-1973 [PMID:19067071 DOI:10.1007/s00464-008-0218-0]
63 Montecucco C,Rappuoli R.Living dangerously:how Helicobacter pylori survives in the human stomach.
2001;2:457-466 [PMID:11389469 DOI:10.1038/35073084]
64 Nagini S.Carcinoma of the stomach:A review of epidemiology,pathogenesis,molecular genetics and chemoprevention.
2012;4:156-169 [PMID:22844547 DOI:10.4251/wjgo.v4.i7.156]
65 Helicobacter and Cancer Collaborative Group.Gastric cancer and Helicobacter pylori:a combined analysis of 12 case control studies nested within prospective cohorts.
2001;49:347-353 [PMID:11511555 DOI:10.1136/gut.49.3.347]
66 Huang JQ,Zheng GF,Sumanac K,Irvine EJ,Hunt RH.Meta-analysis of the relationship between cagA seropositivity and gastric cancer.
2003;125:1636-1644 [PMID:14724815 DOI:10.1053/j.gastro.2003.08.033]
67 Blaser MJ,Perez-Perez GI,Kleanthous H,Cover TL,Peek RM,Chyou PH,Stemmermann GN,Nomura A.Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach.
1995;55:2111-2115[PMID:7743510]
68 Fischer W,Püls J,Buhrdorf R,Gebert B,Odenbreit S,Haas R.Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island:essential genes for CagA translocation in host cells and induction of interleukin-8.
2001;42:1337-1348 [PMID:11886563 DOI:10.1046/j.1365-2958.2001.02714.x]
69 Yang ZM,Chen WW,Wang YF.Gene expression profiling in gastric mucosa from Helicobacter pylori-infected and uninfected patients undergoing chronic superficial gastritis.
2012;7:e33030 [PMID:22438889 DOI:10.1371/journal.pone.0033030]
70 Lee YC,Chiang TH,Chou CK,Tu YK,Liao WC,Wu MS,Graham DY.Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence:A Systematic Review and Metaanalysis.
2016;150:1113-1124.e5 [PMID:26836587 DOI:10.1053/j.gastro.2016.01.028]
71 Chiang TH,Chang WJ,Chen SL,Yen AM,Fann JC,Chiu SY,Chen YR,Chuang SL,Shieh CF,Liu CY,Chiu HM,Chiang H,Shun CT,Lin MW,Wu MS,Lin JT,Chan CC,Graham DY,Chen HH,Lee YC.Mass eradication of
to reduce gastric cancer incidence and mortality:a long-term cohort study on Matsu Islands.
2021;70:243-250 [PMID:32792335 DOI:10.1136/gutjnl-2020-322200]
72 Liou JM,Malfertheiner P,Lee YC,Sheu BS,Sugano K,Cheng HC,Yeoh KG,Hsu PI,Goh KL,Mahachai V,Gotoda T,Chang WL,Chen MJ,Chiang TH,Chen CC,Wu CY,Leow AH,Wu JY,Wu DC,Hong TC,Lu H,Yamaoka Y,Megraud F,Chan FKL,Sung JJ,Lin JT,Graham DY,Wu MS,El-Omar EM;Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM).Screening and eradication of
for gastric cancer prevention:the Taipei global consensus.
2020;69:2093-2112 [PMID:33004546 DOI:10.1136/gutjnl-2020-322368]
73 Larsson SC,Bergkvist L,Wolk A.Fruit and vegetable consumption and incidence of gastric cancer:a prospective study.
2006;15:1998-2001 [PMID:17035412 DOI:10.1158/1055-9965.EPI-06-0402]
74 Neugut AI,Hayek M,Howe G.Epidemiology of gastric cancer.
1996;23:281-291[PMID:8658212]
75 Tsugane S.Salt,salted food intake,and risk of gastric cancer:epidemiologic evidence.
2005;96:1-6 [PMID:15649247 DOI:10.1111/j.1349-7006.2005.00006.x]
76 Shikata K,Kiyohara Y,Kubo M,Yonemoto K,Ninomiya T,Shirota T,Tanizaki Y,Doi Y,Tanaka K,Oishi Y,Matsumoto T,Iida M.A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population:the Hisayama study.
2006;119:196-201[PMID:16450397 DOI:10.1002/ijc.21822]
77 Duell EJ,Travier N,Lujan-Barroso L,Clavel-Chapelon F,Boutron-Ruault MC,Morois S,Palli D,Krogh V,Panico S,Tumino R,Sacerdote C,Quirós JR,Sánchez-Cantalejo E,Navarro C,Gurrea AB,Dorronsoro M,Khaw KT,Allen NE,Key TJ,Bueno-de-Mesquita HB,Ros MM,Numans ME,Peeters PH,Trichopoulou A,Naska A,Dilis V,Teucher B,Kaaks R,Boeing H,Schütze M,Regner S,Lindkvist B,Johansson I,Hallmans G,Overvad K,Egeberg R,Tj?nneland A,Lund E,Weiderpass E,Braaten T,Romieu I,Ferrari P,Jenab M,Stenling R,Aune D,Norat T,Riboli E,González CA.Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
2011;94:1266-1275 [PMID:21993435 DOI:10.3945/ajcn.111.012351]
78 Ladeiras-Lopes R,Pereira AK,Nogueira A,Pinheiro-Torres T,Pinto I,Santos-Pereira R,Lunet N.Smoking and gastric cancer:systematic review and meta-analysis of cohort studies.
2008;19:689-701 [PMID:18293090 DOI:10.1007/s10552-008-9132-y]
79 Smyth EC,Capanu M,Janjigian YY,Kelsen DK,Coit D,Strong VE,Shah MA.Tobacco use is associated with increased recurrence and death from gastric cancer.
2012;19:2088-2094 [PMID:22395977 DOI:10.1245/s10434-012-2230-9]
80 Lissowska J,Groves FD,Sobin LH,Fraumeni JF Jr,Nasierowska-Guttmejer A,Radziszewski J,Regula J,Hsing AW,Zatonski W,Blot WJ,Chow WH.Family history and risk of stomach cancer in Warsaw,Poland.
1999;8:223-227 [PMID:10443951 DOI:10.1097/00008469-199906000-00010]
81 Freeman C,Berg JW,Cutler SJ.Occurrence and prognosis of extranodal lymphomas.
1972;29:252-260 [PMID:5007387 DOI:10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#]
82 Dodd GD.Lymphoma of the hollow abdominal viscera.
1990;28:771-783[PMID:2190270]
83 Herrmann R,Panahon AM,Barcos MP,Walsh D,Stutzman L.Gastrointestinal involvement in non-Hodgkin's lymphoma.
1980;46:215-222 [PMID:7388763 DOI:10.1002/1097-0142(19800701)46:1<215::aid-cncr2820460136>3.0.co;2-6]
84 Ferrucci PF,Zucca E.Primary gastric lymphoma pathogenesis and treatment:what has changed over the past 10 years?
2007;136:521-538 [PMID:17156403 DOI:10.1111/j.1365-2141.2006.06444.x]
85 DARLING RC,WELCH CE.Tumors of the small intestine.
1959;260:397-408[PMID:13632900 DOI:10.1056/NEJM195902262600901]
86 Naqvi MS,Burrows L,Kark AE.Lymphoma of the gastrointestinal tract:prognostic guides based on 162 cases.
1969;170:221-231 [PMID:5819579 DOI:10.1097/00000658-196908000-00010]
87 Bush RS.Primary lymphoma of the gastrointestinal tract.
1974;228:1291-1294 [PMID:4406525]
88 Schottenfeld D,Beebe-Dimmer JL,Vigneau FD.The epidemiology and pathogenesis of neoplasia in the small intestine.
2009;19:58-69 [PMID:19064190 DOI:10.1016/j.annepidem.2008.10.004]
89 Dionigi G,Annoni M,Rovera F,Boni L,Villa F,Castano P,Bianchi V,Dionigi R.Primary colorectal lymphomas:review of the literature.
2007;16 Suppl 1:S169-S171 [PMID:18024019 DOI:10.1016/j.suronc.2007.10.021]
90 Jinnai D,Iwasa Z,Watanuki T.Malignant lymphoma of the large intestine--operative results in Japan.
1983;13:331-336 [PMID:6645123 DOI:10.1007/BF02469515]
91 Kim YH,Lee JH,Yang SK,Kim TI,Kim JS,Kim HJ,Kim JI,Kim SW,Kim JO,Jung IK,Jung SA,Jung MK,Kim HS,Myung SJ,Kim WH,Rhee JC,Choi KY,Song IS,Hyun JH,Min YI.Primary colon lymphoma in Korea:a KASID (Korean Association for the Study of Intestinal Diseases) Study.
2005;50:2243-2247 [PMID:16416168 DOI:10.1007/s10620-005-3041-7]
92 Ghimire P,Wu GY,Zhu L.Primary gastrointestinal lymphoma.
2011;17:697-707 [PMID:21390139 DOI:10.3748/wjg.v17.i6.697]
93 Jaffe ES,Harris NL,Stein H,Isaacson PG.Classification of lymphoid neoplasms:the microscope as a tool for disease discovery.
2008;112:4384-4399 [PMID:19029456 DOI:10.1182/blood-2008-07-077982]
94 Lee HJ,Han JK,Kim TK,Kim YH,Kim AY,Kim KW,Choi JY,Choi BI.Primary colorectal lymphoma:spectrum of imaging findings with pathologic correlation.
2002;12:2242-2249 [PMID:12195476 DOI:10.1007/s00330-002-1307-4]
95 Parsonnet J,Hansen S,Rodriguez L,Gelb AB,Warnke RA,Jellum E,Orentreich N,Vogelman JH,Friedman GD.Helicobacter pylori infection and gastric lymphoma.
1994;330:1267-1271 [PMID:8145781 DOI:10.1056/NEJM199405053301803]
96 Wotherspoon AC,Ortiz-Hidalgo C,Falzon MR,Isaacson PG.Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma.
1991;338:1175-1176 [PMID:1682595 DOI:10.1016/0140-6736(91)92035-z]
97 Ishikura N,Usui Y,Ito H,Kasugai Y,Oze I,Kato S,Yatabe Y,Nakamura S,Matsuo K.Helicobacter pylori (HP) infection alone,but not HP-induced atrophic gastritis,increases the risk of gastric lymphoma:a case-control study in Japan.
2019;98:1981-1987 [PMID:31177299 DOI:10.1007/s00277-019-03721-y]
98 Roggero E,Zucca E,Pinotti G,Pascarella A,Capella C,Savio A,Pedrinis E,Paterlini A,Venco A,Cavalli F.Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue.
1995;122:767-769 [PMID:7717599 DOI:10.7326/0003-4819-122-10-199505150-00006]
99 Rollinson S,Levene AP,Mensah FK,Roddam PL,Allan JM,Diss TC,Roman E,Jack A,MacLennan K,Dixon MF,Morgan GJ.Gastric marginal zone lymphoma is associated with polymorphisms in genes involved in inflammatory response and antioxidative capacity.
2003;102:1007-1011 [PMID:12676777 DOI:10.1182/blood-2002-12-3803]
100 Ahmed N,Sechi LA.Helicobacter pylori and gastroduodenal pathology:new threats of the old friend.
2005;4:1 [PMID:15634357 DOI:10.1186/1476-0711-4-1]
101 Matsumoto T,Shimizu M,Iida M,Amano K,Nakamura S,Fujishima M.Primary low-grade,Bcell,mucosa-associated lymphoid tissue lymphoma of the colorectum:clinical and colonoscopic features in six cases.
1998;48:501-508 [PMID:9831839 DOI:10.1016/s0016-5107(98)70092-6]
102 Grünberger B,W?hrer S,Streubel B,Formanek M,Petkov V,Puespoek A,Haefner M,Hejna M,Jaeger U,Chott A,Raderer M.Antibiotic treatment is not effective in patients infected with Helicobacter pylori suffering from extragastric MALT lymphoma.
2006;24:1370-1375 [PMID:16549831 DOI:10.1200/JCO.2005.02.9025]
103 Cavalli F,Isaacson PG,Gascoyne RD,Zucca E.MALT Lymphomas.
2001;241-258 [PMID:11722987 DOI:10.1182/asheducation-2001.1.241]
104 Dinarello CA.Biologic basis for interleukin-1 in disease.
1996;87:2095-2147 [PMID:8630372]
105 Locksley RM,Killeen N,Lenardo MJ.The TNF and TNF receptor superfamilies:integrating mammalian biology.
2001;104:487-501 [PMID:11239407 DOI:10.1016/s0092-8674(01)00237-9]
106 Martelli M,Ferreri AJ,Agostinelli C,Di Rocco A,Pfreundschuh M,Pileri SA.Diffuse large B-cell lymphoma.
2013;87:146-171 [PMID:23375551 DOI:10.1016/j.critrevonc.2012.12.009]
107 Keum N,Giovannucci E.Global burden of colorectal cancer:emerging trends,risk factors and prevention strategies.
2019;16:713-732 [PMID:31455888 DOI:10.1038/s41575-019-0189-8]
108 Siegel RL,Miller KD,Goding Sauer A,Fedewa SA,Butterly LF,Anderson JC,Cercek A,Smith RA,Jemal A.Colorectal cancer statistics,2020.
2020;70:145-164 [PMID:32133645 DOI:10.3322/caac.21601]
109 Khuhaprema T,Srivatanakul P.Colon and rectum cancer in Thailand:an overview.
2008;38:237-243 [PMID:18356191 DOI:10.1093/jjco/hyn020]
110 Dolatkhah R,Somi MH,Bonyadi MJ,Asvadi Kermani I,Farassati F,Dastgiri S.Colorectal cancer in iran:molecular epidemiology and screening strategies.
2015;2015:643020[PMID:25685149 DOI:10.1155/2015/643020]
111 Ibrahim EM,Zeeneldin AA,El-Khodary TR,Al-Gahmi AM,Bin Sadiq BM.Past,present and future of colorectal cancer in the Kingdom of Saudi Arabia.
2008;14:178-182 [PMID:19568534 DOI:10.4103/1319-3767.43275]
112 International Agency for Research on Cancer.Globocan 2018:Cancer Fact Sheets — Colorectal Cancer.IARC.[cited 3 March 2021].Available from:http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf
113 Hu KC,Wu MS,Chu CH,Wang HY,Lin SC,Liu SC,Liu CC,Su TH,Chen CL,Liu CJ,Shih SC.Synergistic Effect of Hyperglycemia and Helicobacterpylori Infection Status on Colorectal Adenoma Risk.
2017;102:2744-2750 [PMID:28475740 DOI:10.1210/jc.2017-00257]
114 NCD Risk Factor Collaboration (NCD-RisC).Worldwide trends in diabetes since 1980:a pooled analysis of 751 population-based studies with 4.4 million participants.
2016;387:1513-1530[PMID:27061677 DOI:10.1016/S0140-6736(16)00618-8]
115 Dow LE,O'Rourke KP,Simon J,Tschaharganeh DF,van Es JH,Clevers H,Lowe SW.Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer.
2015;161:1539-1552 [PMID:26091037 DOI:10.1016/j.cell.2015.05.033]
116 Armaghany T,Wilson JD,Chu Q,Mills G.Genetic alterations in colorectal cancer.
2012;5:19-27 [PMID:22574233]
117 Kedrin D,Gala MK.Genetics of the serrated pathway to colorectal cancer.
2015;6:e84 [PMID:25856207 DOI:10.1038/ctg.2015.12]
118 O'Brien MJ,Yang S,Mack C,Xu H,Huang CS,Mulcahy E,Amorosino M,Farraye FA.Comparison of microsatellite instability,CpG island methylation phenotype,BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points.
2006;30:1491-1501 [PMID:17122504 DOI:10.1097/01.pas.0000213313.36306.85]
119 Kim KM,Lee EJ,Ha S,Kang SY,Jang KT,Park CK,Kim JY,Kim YH,Chang DK,Odze RD.Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea.
2011;35:1274-1286 [PMID:21836485 DOI:10.1097/PAS.0b013e318224cd2e]
120 Meucci G,Tatarella M,Vecchi M,Ranzi ML,Biguzzi E,Beccari G,Clerici E,de Franchis R.High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas.
1997;25:605-607 [PMID:9451672 DOI:10.1097/00004836-199712000-00011]
121 Breuer-Katschinski B,Nemes K,Marr A,Rump B,Leiendecker B,Breuer N,Goebell H.Helicobacter pylori and the risk of colonic adenomas.Colorectal Adenoma Study Group.
1999;60:210-215 [PMID:10343134 DOI:10.1159/000007661]
122 Sonnenberg A,Genta RM.Helicobacter pylori is a risk factor for colonic neoplasms.
2013;108:208-215 [PMID:23208272 DOI:10.1038/ajg.2012.407]
123 Rokkas T,Sechopoulos P,Pistiolas D,Kothonas F,Margantinis G,Koukoulis G.The relationship of Helicobacter pylori infection and colon neoplasia,on the basis of meta-analysis.
2013;25:1286-1294 [PMID:23820245 DOI:10.1097/MEG.0b013e328363d3cd]
124 Liou JM,Lin JW,Huang SP,Lin JT,Wu MS.Helicobacter pylori infection is not associated with increased risk of colorectal polyps in Taiwanese.
2006;119:1999-2000 [PMID:16708392 DOI:10.1002/ijc.22050]
125 Machida-Montani A,Sasazuki S,Inoue M,Natsukawa S,Shaura K,Koizumi Y,Kasuga Y,Hanaoka T,Tsugane S.Atrophic gastritis,Helicobacter pylori,and colorectal cancer risk:a casecontrol study.
2007;12:328-332 [PMID:17669106 DOI:10.1111/j.1523-5378.2007.00513.x]
126 Choi DS,Seo SI,Shin WG,Park CH.Risk for Colorectal Neoplasia in Patients With Helicobacter pylori Infection:A Systematic Review and Meta-analysis.
2020;11:e00127 [PMID:32032128 DOI:10.14309/ctg.0000000000000127]
127 Zuo Y,Jing Z,Bie M,Xu C,Hao X,Wang B.Association between Helicobacter pylori infection and the risk of colorectal cancer:A systematic review and meta-analysis.
2020;99:e21832 [PMID:32925719 DOI:10.1097/MD.0000000000021832]
128 Grahn N,Hmani-Aifa M,Fransén K,S?derkvist P,Monstein HJ.Molecular identification of Helicobacter DNA present in human colorectal adenocarcinomas by 16S rDNA PCR amplification and pyrosequencing analysis.
2005;54:1031-1035 [PMID:16192433 DOI:10.1099/jmm.0.46122-0]
129 Soylu A,Ozkara S,Alis H,Dolay K,Kalayci M,Yasar N,Kumbasar AB.Immunohistochemical testing for Helicobacter Pylori existence in neoplasms of the colon.
2008;8:35[PMID:18702825 DOI:10.1186/1471-230X-8-35]
130 Wong SH,Yu J.Gut microbiota in colorectal cancer:mechanisms of action and clinical applications.
2019;16:690-704 [PMID:31554963 DOI:10.1038/s41575-019-0209-8]
131 Hua S,Marks E,Schneider JJ,Keely S.Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease:selective targeting to diseased versus healthy tissue.
2015;11:1117-1132 [PMID:25784453 DOI:10.1016/j.nano.2015.02.018]
132 Butt J,Epplein M.Helicobacter pylori and colorectal cancer-A bacterium going abroad?
2019;15:e1007861 [PMID:31393968 DOI:10.1371/journal.ppat.1007861]
133 Hu KC,Wu MS,Chu CH,Wang HY,Lin SC,Liu CC,Su TH,Liao WC,Chen CL,Liu CJ,Shih SC.Decreased Colorectal Adenoma Risk After Helicobacter pylori Eradication:A Retrospective Cohort Study.
2019;68:2105-2113 [PMID:30566695 DOI:10.1093/cid/ciy591]
134 Ahmed RL,Schmitz KH,Anderson KE,Rosamond WD,Folsom AR.The metabolic syndrome and risk of incident colorectal cancer.
2006;107:28-36 [PMID:16721800 DOI:10.1002/cncr.21950]
135 Haggar FA,Boushey RP.Colorectal cancer epidemiology:incidence,mortality,survival,and risk factors.
2009;22:191-197 [PMID:21037809 DOI:10.1055/s-0029-1242458]
136 Lieberman DA,Prindiville S,Weiss DG,Willett W;VA Cooperative Study Group 380.Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals.
2003;290:2959-2967 [PMID:14665657 DOI:10.1001/jama.290.22.2959]
137 Tseng PH,Lee YC,Chiu HM,Chen CC,Liao WC,Tu CH,Yang WS,Wu MS.Association of diabetes and HbA1c levels with gastrointestinal manifestations.
2012;35:1053-1060[PMID:22410812 DOI:10.2337/dc11-1596]
138 Yuhara H,Steinmaus C,Cohen SE,Corley DA,Tei Y,Buffler PA.Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer?
2011;106:1911-21;quiz 1922 [PMID:21912438 DOI:10.1038/ajg.2011.301]
139 Tsong WH,Koh WP,Yuan JM,Wang R,Sun CL,Yu MC.Cigarettes and alcohol in relation to colorectal cancer:the Singapore Chinese Health Study.
2007;96:821-827 [PMID:17311023 DOI:10.1038/sj.bjc.6603623]
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
