Impact of the microenvironment on the pathogenesis of mucosaassociated lymphoid tissue lymphomas
INTRODUCTION
Extranodal mucosa-associated lymphoid tissue (MALT) lymphomas account for 5%-8% of all non-Hodgkin lymphomas (NHLs) and were first described in 1983 by Isaacson and Wright[1-3].MALT lymphomas arise at a wide range of extranodal sites,most frequently occurring in the stomach,followed by the lung,ocular adnexa,thyroid and small intestine[4].The cells of this type of lymphoma have the same cytological and immunophenotypical (CD20+,CD21+,CD35+,IgM+,and IgD-)features as marginal zone B cells,prompting the World Health Organization to designate this lymphoma “extranodal marginal zone B cell lymphoma of mucosaassociated lymphoid tissue (MALT lymphoma)”[5].The cell of origin of MALT lymphomas is the marginal zone (MZ) B cell.These B cells are a first line of defense against infectious agents and build up an innate-like antibody response in a T cellindependent and T cell-dependent manner[6,7].MALT lymphomagenesis is highly dependent on microenvironmental factors and therefore often associated with chronic inflammation induced either by
(
),the most common pathogen in gastric MALT lymphomas,or by chronic inflammation as a result of autoimmune disease.These are known risk factors for the development of MZ lymphomas[8].In addition to the antigenic drive,oncogenic events are important in the process of malignant transformation[8].MALT lymphoma cell proliferation is driven by T cell signaling,chronic (auto) antigen stimulation of MZ B cells,and activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB pathway)[9].
ACQUIRED GENETIC ABNORMALITIES
In MALT lymphomas,recurrent chromosomal aberrations,such as trisomies,amplifications and deletions,chromosomal translocations,somatic point mutations,and promotor hypermethylation,have been described.
Return to place in story. 3. He cut out the shoes which he wished to begin to make the next morning: Note that while the shoemaker receives magical help, he still does part of the work himself, buying, preparing, and cutting the leather for the shoes each evening. He doesn t wait lazily for all the work to be done for him nor does he give up when life appears hopeless at the beginning of the tale.Return to place in story. 4. Commended himself to God: The Grimms added much of the overt13 religious references in their tales as they edited them for a young audience.Maria Tatar writes:
The most common cytogenetic alterations are trisomies 3,12,and/or 18,which are present in 20%-35% of cases,and they are often associated with one of the four main translocations[10-13].Trisomies 3 and 18 and losses at 6q23 occur in MALT lymphomas primarily involving the stomach,orbital adnexa,thyroid,salivary glands,and lung[13].Several promising candidate genes are located on chromosome 3,such as the proto-oncogene
and the transcription factor
[14]
Additionally,the chemokine receptor
,genomically located on chromosome 3 (3p24),is highly expressed in trisomy 3-positive MALT lymphomas[15].Furthermore,genome-wide DNA profiling revealed deletions in 1p and 6q,as well as gains on chromosomes 3 and 18 and the short arm of chromosome 6[10].
The most common chromosomal translocations associated with the pathogenesis of MALT lymphomas are t(1;14)(p22,q32) (involving the
and
genes),t(11;18)(q21,q21) (involving BIRC3/MALT1),t(14;18)(q32,q21) (involving
/
)and t(3;14)(p14.1,q32) (involving
-
)[8,10,16].The frequency of genetic aberrations is dependent on the primary site of disease[10,17].At least three translocations,t(11;18),t(14;18) and t(1;14),involve the
and
genes and lead to activation of the NF-кB pathway in lymphocytes,thus indicating that these aberrations are oncogenic events[18,19].
It is well known that MALT lymphomas are commonly associated with long-lasting chronic inflammation caused by microbial pathogens and/or autoimmune diseases that trigger sustained lymphoid proliferation.The low activation threshold of MZ B cells may predispose them to neoplastic transformation[29].
Then Beauty asked her father what he thought could be the meaning of her strange dreams, and why the Prince constantly begged her not to trust to appearances
Sometime later, years later, my grandmother gave me the clock and the key. The old house was quiet. No bowls clanging, no laughter over the dinner table, no ticking or chiming of the clock-all was still. The hands on the clock were frozen, a reminder7 of time slipping away, stopped at the precise moment when my grandfather had ceased winding it. I took the key in my shaking hand and opened the clock door. All of a sudden, I was a child again, watching my grandfather with his silver-white hair and twinkling blue eyes. He was there, winking8 at me, at the secret of the clock s magic, at the key that held so much power. I stood, lost in the moment for a long time. Then slowly, reverently9, I inserted the key and wound the clock. It sprang to life. Tick-tock, tick-tock, life and chimes were breathed into the dining room, into the house and into my heart. In the movement of the hands of the clock, my grandfather lived again.
Aberrant somatic hypermutation is associated with genetic lesions in malt lymphomas
Aberrant somatic hypermutation (ASHM) has been identified to be crucial for the development of lymphoid neoplasms.ASHM occurs commonly in diffuse large B cell lymphomas but is rare in indolent lymphomas[10,25,26].The pathogenesis of most lymphomas is associated with distinct genetic lesions arising from mistakes during class switch recombination (CSR) and somatic hypermutation (SHM)[10,27].Activation-induced cytidine deaminase (AID) is an enzyme required for CSR and SHM.Mistargeting of AID to known proto-oncogenes combined with a breakdown of protective high-fidelity repair mechanisms has been shown to be a principal contributor to the pathogenesis of B-NHL[10].Our research group has demonstrated that the expression levels of AID are associated with the mutational load caused by ASHM in MALT lymphomas[25].However,the mechanism causing the upregulation of AID has not been identified thus far.It has been demonstrated that
infection upregulates AID expression
NF-κB in gastric cells
and
,resulting in the accumulation of
mutations[28].Hence,it might be speculated that
infection is also participates in the upregulation of AID in B cells,leading to the accumulation of genetic alterations.
CHRONIC INFLAMMATION SHAPES THE MICROENVIRONMENT AND THEREBY PLAYS A KEY ROLE IN MALT LYMPHOMAGENESIS
We observed somatic missense mutations in
and
in 46% of gastric and 30% of extragastric MALT lymphomas[20].In addition,missense and frameshift mutations in
were described in 20.8% of MALT lymphomas (mainly of gastric origin)[10].Moreover,whole exome sequencing of extragastric MALT lymphomas identified recurrent novel somatic mutations in
,
,and
and in two G protein-coupled receptors (
and
),which have not been reported to be somatically mutated in human tumors thus far.In addition,recurrent mutations were found in two genes (
and
),for which somatic mutations were already reported in ocular adnexal MALT lymphomas.The mutation frequencies of these genes were remarkably variable among MALT lymphomas affecting different sites[21].Sequencing of NF-κB signaling pathway-related genes —
,
,
,and
,known to be frequently mutated in aggressive lymphomas[10,22]— demonstrated that 6% of MALT lymphoma cases exhibited missense or frameshift mutations in the
locus.A total of 28.6% of the ocular adnexal MALT lymphomas had mutations in the
locus[10,23].
and
were not affected in ocular adnexal MALT lymphomas[10].
Gastric MALT lymphomas show a strong association with chronic
infection[30].Other infectious associations have been reported for
(skin)[31],
(intestine)[32],
(lung)[33],
(ocular,nongastrointestinal MALT lymphomas)[34-36] and hepatitis C virus(splenic marginal zone lymphoma)[37].The strength of these associations shows vast geographical discrepancies[38-40].In addition,an association of MALT lymphomas with chronic inflammation induced by autoimmune disease is found in primary Sj?gren’s syndrome (pSS)[41-43] and Hashimoto thyroiditis[44].
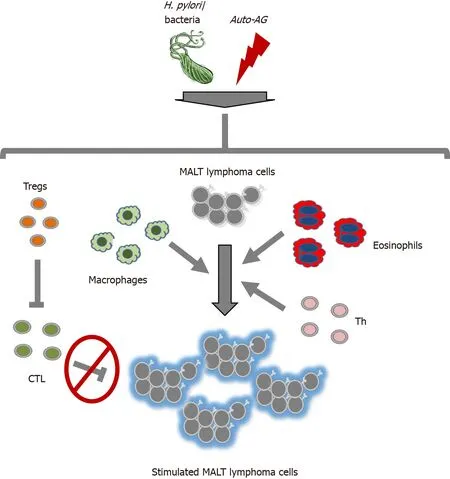
Long-lasting chronic inflammation,
,induced by
infection or pSS,is the trigger for a multistage process in the evolution of MALT lymphomas due direct effects on B cell proliferation and/or survival and/or indirect effects on the activation of innate and adaptive immune cells[9,43,45] as shown in Figure 1.
As already described,MALT lymphomas with long-lasting chronic infections cause B cell proliferation and/or survival either directly and/or indirectly
activation of immune cells[9,43,45,66].Therefore,these interactions provide multiple potential targets for new immunomodulatory treatments beyond the established treatment options for
eradication by antibiotics,radiation,chemotherapy and treatment with the anti-CD20 antibody therapy rituximab[67].
strains expressing cytotoxin-associated gene A (CagA) are associated with the lymphomagenesis of gastric MALT lymphoma[46,47].CagA is involved in the promotion of proliferation and the inhibition of apoptosis of B lymphocytes through activation of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) and upregulation of BCL-2 and BCL-xL[47].Second,the CagA tyrosine phosphorylation-independent pathway impairs p53
AKT serine/threonine kinase 1 (AKT1) and human homolog of double minute 2 (HDM2)[9,45].In general,cell wall lipopolysaccharide has been shown to be responsible for triggering a pattern of mucosal inflammation
Toll-like receptor signaling,resulting in activation of MAPK,phosphoinositide 3-kinase (PI3K) and NF-κB pathways in
infection[48,49].
And when they had driven the stakes into the ground, and had made the walls of the hut, the little hare told Big Lion to climb upon the top while he stayed inside
Further therapeutic targets are related to Tregs,which are recruited into the microenvironment of MALT lymphoma[58,72] and suppress antitumoral immune reactions[58,60,73,74].It has been shown that the Bruton’s kinase inhibitor ibrutinib reduces the number of Tregs in the early course of treatment in chronic lymphocytic leukemia (CLL),in addition to inhibiting the BCR pathway[75].Ibrutinib has been tested in relapsed/refractory marginal zone B cell lymphoma (MZL) and possesses a remarkable response rate with tolerable toxicity[53].However,no data are available thus far for the treatment of MALT lymphoma.
It has been demonstrated that T cells,macrophages and neutrophils recruited during long-lasting chronic inflammation contribute to the formation of genetic aberrations,DNA damage and genetic instability in B cells,leading to antigenindependent lymphoma cell growth.These effects are mediated by activation of ASHM and class-switching recombination in MALT lymphomas[63] and are associated with epigenetic and genetic changes in p57
[24],p16
[24,64] and p53[10] as well as chromosomal translocation of
and
[10,65].
TUMOR MICROENVIRONMENT-TARGETING THERAPIES
He gathered slowly, moving backwards4 and forwards through the bushes, but always keeping up with her. After a while, he came back to the path. She answered his questions - the children, her job, her parents - but always skirted round the main issue.
I read you poetry from Robbie Burns and Walt Whitman, and rubbed lotion4 on your hands. Finally, I worked up the courage to sing to you again. You weren t able to ask me this time. Grandma peeked5 through the door and gave us a tearful smile. I stopped. Keep singing to your mother, she said. When I finished Dad asked me, Would you sing at the memorial service? You were lying right beside me, and suddenly it seemed so perverse6 to have this conversation in front of you. I don t know if I can. I ll try. We didn t speak of it again.
Immunomodulatory drugs (IMiDs) represent a novel therapeutic approach to target the tumor microenvironment of MALT lymphomas.IMiDs,consisting of thalidomide,lenalidomide and pomalidomide,are approved for the treatment of multiple myeloma,and lenalidomide is approved for the treatment of relapsed follicular lymphoma[53,68-70].IMiDs exert anti-inflammatory effects,such as decreased production of cytokines and increased production of Th1 type cytokines;furthermore,they decrease vascular endothelial growth factor (VEGF) levels and show modulating effects on basic cellular mechanisms (T cell costimulation and alteration of FOXP3+Tregs and natural killer cells)[68,69].The efficacy of lenalidomide in MALT lymphomas has been reported in studies with induction of remission after treatment for up to 32 mo[71].Raderer and Kiesewetter[53] conducted a phase II study with a combination therapy consisting of lenalidomide and rituximab,which achieved an overall response rate of 80% and a complete remission rate of 54%.
As already mentioned,long-lasting chronic inflammatory processes might also influence MALT lymphomagenesis through the direct (auto) antigen-mediated interaction of lymphoma cells with immune cells and/or the secretion of soluble factors like cytokines.In this case,a direct immune cell-lymphoma cell interaction and subsequent activation result.Activated T cells targeting
represent cells targeting autoantigens in the case of pSS,and these T cells are present in MALT lymphomas and are able to promote lymphoma cell growth
CD40-mediated signaling and T helper (Th) type-2 cytokine (IL-4,IL-5,and IL-10) effects[50,51].Examples of two cytokines are a proliferation-inducing ligand (APRIL) and B cellactivating factor (BAFF),which are members of the tumor necrosis factor family and play a key role in B cells and autoimmunity.Both cytokines are secreted by eosinophils and/or macrophages and stimulate MALT lymphoma cells[52-54].Both the CD40/CD40L interaction and APRIL and/or BAFF signaling cause the activation of important downstream signaling pathways,
,NF-κB and/or MAPK[55,56],and thereby have an important impact on MALT lymphomagenesis.
Chronic inflammatory processes in MALT lymphomas not only promote B cell growth/proliferation but also actively induce immunosuppressive conditions,which also play a major role in the development and progression of this B cell malignancy.These effects are partially mediated by recruited regulatory T cells (Tregs)[57,58],which suppress anticancer immunity by secreting anti-inflammatory cytokines and/or expressing immune inhibitory surface receptors[59,60].Furthermore,activated tumorinfiltrating T cells have dysfunctional cytolytic capacity in MALT lymphomas[61,62].
As reported in section 3,in MALT lymphoma cells,the NF-κB pathway is strongly activated by genetic alterations[18,76,77] or by interaction with activated immune cells
the CD40/CD40L[52-54,56] and/or APRIL axes[77-79].
Finally,promoter hypermethylation of the tumor suppressor genes
and
has been reported in low-grade MALT lymphoma cases[24].CpG hypermethylation of
has been detected in 26% of investigated MALT lymphomas,including ocular adnexal cases and lymphomas located in the salivary and thyroid glands[10].
Bortezomib,a proteasome inhibitor with inhibitory effects on the NF-κB signaling pathway[10],showed promising response rates in MALT lymphoma patients in phase II trials[80].Furthermore,bortezomib was reported to reverses the tumor-induced dysfunction of CD8+ T cells by increasing the expression of Notch cascade genes[81].Moreover,bortezomib enacts immunostimulatory effects by activating tumor-infiltrating CD8+ T cells[61,62].Taken together,these findings suggest that the antilymphoma effects of bortezomib are mediated by NF-κB inhibition and by reversal of the observed T cell malfunction[52-54].
23. By the hand: This is one of the few popular tales in which two siblings81 work together with affection and concern. Another tale is Snow White and Rose Red in which the two sisters are described as often exploring the forest hand in hand.Return to place in story.
Another possibility to suppress NF-κB activation in MALT lymphoma cells is the disruption of the APRIL axis[82] with use of an anti-APRIL antibody;one such antibody was developed by Guadagnoli
[82] and has shown promising results in CLL in a preclinical setting[83-89].However,this strategy has not been tested in MALT lymphoma patients thus far.
It has also been demonstrated that macrolides,which are used for eradication of bacterial infection in MALT lymphomas,have certain immunomodulatory effects,
,they decrease the number and inhibit the function of neutrophils as well as eosinophils and inhibit Th2 cell functions[83-89].Thus,it is likely that the immunomodulatory effects significantly impact the response rates of MALT lymphomas when these antimicrobial drugs are used.
CONCLUSION
MALT lymphomas represent a heterogeneous group of lymphoid neoplasms arising at different extranodal sites and are associated with a variety of long-lasting chronic infections.In the current pathogenic model,(auto) antigen stimuli trigger lymphoma cell growth,survival,and recruitment of immune cells to the microenvironment,which in turn stimulate lymphoma cells directly
surface receptor interactions and/or indirectly
cytokine secretion.Moreover,it has been shown that inflammatory processes may lead to the acquisition of further genetic alterations resulting in lymphoma cell growth independent of (auto) antigen stimuli.Many agents targeting/blocking the interaction of immune cells of the microenvironment with lymphoma cells,as well as eradicating the antigen stimuli,have been developed within recent years,indicating that the basis for novel therapeutic strategies is already available.Despite these advances,the number of comprehensive studies on the microenvironment composition and its interaction with lymphoma cells needs to be significantly increased to gain further knowledge on targets for innovative and efficient therapy.
We thank Waha JE for reviewing the manuscript for clarity in English as a native speaker.
1 Isaacson P,Wright DH.Malignant lymphoma of mucosa-associated lymphoid tissue.A distinctive type of B-cell lymphoma.
1983;52:1410-1416 [PMID:6193858 DOI:10.1002/1097-0142(19831015)52:8<1410::AID-CNCR2820520813>3.0.CO;2-3]
2 A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma.The Non-Hodgkin's Lymphoma Classification Project.
1997;89:3909-3918[PMID:9166827 DOI:10.1182/blood.v89.11.3909]
3 Olszewski AJ,Castillo JJ.Survival of patients with marginal zone lymphoma:analysis of the Surveillance,Epidemiology,and End Results database.
2013;119:629-638 [PMID:22893605 DOI:10.1002/cncr.27773]
4 Isaacson PG,Du MQ.MALT lymphoma:from morphology to molecules.
2004;4:644-653 [PMID:15286744 DOI:10.1038/nrc1409]
5 Swerdlow SH,Campo E,Harris NL,Jaffe ES,Pileri SA,Stein H.Weltgesundheitsorganisation.WHO classification of tumours of haematopoietic and lymphoid tissues.[cited 12 January 2021].In:International Agency for Research on Cancer [Internet].Available from:https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHOClassification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017
6 Efremov DG,Turkalj S,Laurenti L.Mechanisms of B Cell Receptor Activation and Responses to B Cell Receptor Inhibitors in B Cell Malignancies.
2020;12 [PMID:32481736 DOI:10.3390/cancers12061396]
7 Cerutti A,Cols M,Puga I.Marginal zone B cells:virtues of innate-like antibody-producing lymphocytes.
2013;13:118-132 [PMID:23348416 DOI:10.1038/nri3383]
8 Zucca E,Bertoni F,Vannata B,Cavalli F.Emerging role of infectious etiologies in the pathogenesis of marginal zone B-cell lymphomas.
2014;20:5207-5216 [PMID:25320370 DOI:10.1158/1078-0432.CCR-14-0496]
9 Kiesewetter B,Raderer M.Immunomodulatory treatment for mucosa-associated lymphoid tissue lymphoma (MALT lymphoma).
2020;38:417-424 [PMID:32469432 DOI:10.1002/hon.2754]
10 Troppan K,Wenzl K,Neumeister P,Deutsch A.Molecular Pathogenesis of MALT Lymphoma.
2015;2015:102656 [PMID:25922601 DOI:10.1155/2015/102656]
11 Krugmann J,Tzankov A,Dirnhofer S,Fend F,Wolf D,Siebert R,Probst P,Erdel M.Complete or partial trisomy 3 in gastro-intestinal MALT lymphomas co-occurs with aberrations at 18q21 and correlates with advanced disease stage:a study on 25 cases.
2005;11:7384-7385 [PMID:16437648 DOI:10.3748/wjg.v11.i46.7384]
12 Taji S,Nomura K,Matsumoto Y,Sakabe H,Yoshida N,Mitsufuji S,Nishida K,Horiike S,Nakamura S,Morita M,Taniwaki M.Trisomy 3 may predict a poor response of gastric MALT lymphoma to Helicobacter pylori eradication therapy.
2005;11:89-93 [PMID:15609403 DOI:10.3748/wjg.v11.i1.89]
13 Kwee I,Rancoita PM,Rinaldi A,Ferreri AJ,Bhagat G,Gascoyne RD,Canzonieri V,Gaidano G,Doglioni C,Zucca E,Ponzoni M,Bertoni F.Genomic profiles of MALT lymphomas:variability across anatomical sites.
2011;96:1064-1066 [PMID:21459788 DOI:10.3324/haematol.2011.040402]
14 Dierlamm J,Wlodarska I,Michaux L,Stefanova M,Hinz K,Van Den Berghe H,Hagemeijer A,Hossfeld DK.Genetic abnormalities in marginal zone B-cell lymphoma.
2000;18:1-13 [PMID:10797525 DOI:10.1002/(SICI)1099-1069(200003)18:1<1::AID-HON647>3.0.CO;2-G]
15 Deutsch AJ,Aigelsreiter A,Steinbauer E,Frühwirth M,Kerl H,Beham-Schmid C,Schaider H,Neumeister P.Distinct signatures of B-cell homeostatic and activation-dependent chemokine receptors in the development and progression of extragastric MALT lymphomas.
2008;215:431-444 [PMID:18561120 DOI:10.1002/path.2372]
16 Willis TG,Jadayel DM,Du MQ,Peng H,Perry AR,Abdul-Rauf M,Price H,Karran L,Majekodunmi O,Wlodarska I,Pan L,Crook T,Hamoudi R,Isaacson PG,Dyer MJ.Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types.
1999;96:35-45 [PMID:9989495 DOI:10.1016/S0092-8674(00)80957-5]
17 Bertoni F,Zucca E.Delving deeper into MALT lymphoma biology.
2006;116:22-26[PMID:16395399 DOI:10.1172/JCI27476]
18 Lucas PC,Yonezumi M,Inohara N,McAllister-Lucas LM,Abazeed ME,Chen FF,Yamaoka S,Seto M,Nunez G.Bcl10 and MALT1,independent targets of chromosomal translocation in malt lymphoma,cooperate in a novel NF-kappa B signaling pathway.
2001;276:19012-19019 [PMID:11262391 DOI:10.1074/jbc.M009984200]
19 Uren AG,O'Rourke K,Aravind LA,Pisabarro MT,Seshagiri S,Koonin EV,Dixit VM.Identification of paracaspases and metacaspases:two ancient families of caspase-like proteins,one of which plays a key role in MALT lymphoma.
2000;6:961-967 [PMID:11090634 DOI:10.1016/s1097-2765(00)00094-0]
20 Deutsch AJ,Frühwirth M,Aigelsreiter A,Cerroni L,Neumeister P.Primary cutaneous marginal zone B-cell lymphomas are targeted by aberrant somatic hypermutation.
2009;129:476-479 [PMID:18704108 DOI:10.1038/jid.2008.243]
21 Moody S,Thompson JS,Chuang SS,Liu H,Raderer M,Vassiliou G,Wlodarska I,Wu F,Cogliatti S,Robson A,Ashton-Key M,Bi Y,Goodlad J,Du MQ.Novel
and
mutation and distinct genetic profiles in MALT lymphomas of different sites.
2018;103:1329-1336[PMID:29674500 DOI:10.3324/haematol.2018.191601]
22 Lenz G,Davis RE,Ngo VN,Lam L,George TC,Wright GW,Dave SS,Zhao H,Xu W,Rosenwald A,Ott G,Muller-Hermelink HK,Gascoyne RD,Connors JM,Rimsza LM,Campo E,Jaffe ES,Delabie J,Smeland EB,Fisher RI,Chan WC,Staudt LM.Oncogenic CARD11 mutations in human diffuse large B cell lymphoma.
2008;319:1676-1679 [PMID:18323416 DOI:10.1126/science.1153629]
23 Li ZM,Rinaldi A,Cavalli A,Mensah AA,Ponzoni M,Gascoyne RD,Bhagat G,Zucca E,Bertoni F.MYD88 somatic mutations in MALT lymphomas.
2012;158:662-664 [PMID:22640364 DOI:10.1111/j.1365-2141.2012.09176.x]
24 Min KO,Seo EJ,Kwon HJ,Lee EJ,Kim WI,Kang CS,Kim KM.Methylation of p16(INK4A) and p57(KIP2) are involved in the development and progression of gastric MALT lymphomas.
2006;19:141-148 [PMID:16357845 DOI:10.1038/modpathol.3800505]
25 Deutsch AJ,Aigelsreiter A,Staber PB,Beham A,Linkesch W,Guelly C,Brezinschek RI,Fruhwirth M,Emberger W,Buettner M,Beham-Schmid C,Neumeister P.MALT lymphoma and extranodal diffuse large B-cell lymphoma are targeted by aberrant somatic hypermutation.
2007;109:3500-3504 [PMID:17197434 DOI:10.1182/blood-2006-06-030494]
26 B?d?r C,Bognár A,Reiniger L,Szepesi A,Tóth E,Kopper L,Matolcsy A.Aberrant somatic hypermutation and expression of activation-induced cytidine deaminase mRNA in mediastinal large B-cell lymphoma.
2005;129:373-376 [PMID:15842661 DOI:10.1111/J.1365-2141.2005.05454.X]
27 Küppers R,Dalla-Favera R.Mechanisms of chromosomal translocations in B cell lymphomas.
2001;20:5580-5594 [PMID:11607811 DOI:10.1038/sj.onc.1204640]
28 Matsumoto Y,Marusawa H,Kinoshita K,Endo Y,Kou T,Morisawa T,Azuma T,Okazaki IM,Honjo T,Chiba T.Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium.
2007;13:470-476 [PMID:17401375 DOI:10.1038/nm1566]
29 Isaacson PG,Spencer J.The biology of low grade MALT lymphoma.
1995;48:395-397 [PMID:7629280 DOI:10.1136/jcp.48.5.395]
30 Wotherspoon AC,Ortiz-Hidalgo C,Falzon MR,Isaacson PG.Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma.
1991;338:1175-1176 [PMID:1682595 DOI:10.1016/0140-6736(91)92035-Z]
31 Cerroni L,Z?chling N,Pütz B,Kerl H.Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma.
1997;24:457-461 [PMID:9331890 DOI:10.1111/j.1600-0560.1997.tb01318.x]
32 Parsonnet J,Isaacson PG.Bacterial infection and MALT lymphoma.
2004;350:213-215 [PMID:14724298 DOI:10.1056/nejmp038200]
33 Adam P,Czapiewski P,Colak S,Kosmidis P,Tousseyn T,Sagaert X,Boudova L,Okoń K,Morresi-Hauf A,Agostinelli C,Pileri S,Pruneri G,Martinelli G,Du MQ,Fend F.Prevalence of Achromobacter xylosoxidans in pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe.
2014;164:804-810 [PMID:24372375 DOI:10.1111/bjh.12703]
34 Ferreri AJ,Guidoboni M,Ponzoni M,De Conciliis C,Dell'Oro S,Fleischhauer K,Caggiari L,Lettini AA,Dal Cin E,Ieri R,Freschi M,Villa E,Boiocchi M,Dolcetti R.Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas.
2004;96:586-594[PMID:15100336 DOI:10.1093/jnci/djh102]
35 Chanudet E,Adam P,Nicholson AG,Wotherspoon AC,Ranaldi R,Goteri G,Pileri SA,Ye H,Müller-Hermelink HK,Du MQ.Chlamydiae and Mycoplasma infections in pulmonary MALT lymphoma.
2007;97:949-951 [PMID:17876330 DOI:10.1038/sj.bjc.6603981]
36 Aigelsreiter A,Gerlza T,Deutsch AJ,Leitner E,Beham-Schmid C,Beham A,Popper H,Borel N,Pospischil A,Raderer M,Kessler HH,Neumeister P.Chlamydia psittaci Infection in nongastrointestinal extranodal MALT lymphomas and their precursor lesions.
2011;135:70-75 [PMID:21173126 DOI:10.1309/AJCPXMDRT1SY6KIV]
37 Negri E,Little D,Boiocchi M,La Vecchia C,Franceschi S.B-cell non-Hodgkin's lymphoma and hepatitis C virus infection:a systematic review.
2004;111:1-8 [PMID:15185336 DOI:10.1002/ijc.20205]
38 Aoyama S,Masaki A,Sakamoto Y,Takino H,Murase T,Ohshima K,Yoshino T,Kato S,Inagaki H.Achromobacter Infection Is Rare in Japanese Patients with Pulmonary B-cell Lymphoma.
2018;57:789-794 [PMID:29151525 DOI:10.2169/internalmedicine.9430-17]
39 Carugi A,Onnis A,Antonicelli G,Rossi B,Mannucci S,Luzzi A,Lazzi S,Bellan C,Tosi GM,Sayed S,De Falco G,Leoncini L.Geographic variation and environmental conditions as cofactors in Chlamydia psittaci association with ocular adnexal lymphomas:a comparison between Italian and African samples.
2010;28:20-26 [PMID:19728399 DOI:10.1002/hon.921]
40 Chanudet E,Zhou Y,Bacon CM,Wotherspoon AC,Müller-Hermelink HK,Adam P,Dong HY,de Jong D,Li Y,Wei R,Gong X,Wu Q,Ranaldi R,Goteri G,Pileri SA,Ye H,Hamoudi RA,Liu H,Radford J,Du MQ.Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions.
2006;209:344-351 [PMID:16583361 DOI:10.1002/path.1984]
41 W?hrer S,Troch M,Streubel B,Zwerina J,Skrabs C,Formanek M,Hauff W,Hoffmann M,Müllauer L,Chott A,Raderer M.MALT lymphoma in patients with autoimmune diseases:a comparative analysis of characteristics and clinical course.
2007;21:1812-1818 [PMID:17554381 DOI:10.1038/sj.leu.2404782]
42 Ferraccioli GF,Sorrentino D,De Vita S,Casatta L,Labombarda A,Avellini C,Dolcetti R,Di Luca D,Beltrami CA,Boiocchi M,Bartoli E.B cell clonality in gastric lymphoid tissues of patients with Sj?gren's syndrome.
1996;55:311-316 [PMID:8660105 DOI:10.1136/ard.55.5.311]
43 Nocturne G,Mariette X.Sj?gren Syndrome-associated lymphomas:an update on pathogenesis and management.
2015;168:317-327 [PMID:25316606 DOI:10.1111/bjh.13192]
44 Troch M,Woehrer S,Streubel B,Weissel M,Hoffmann M,Müllauer L,Chott A,Raderer M.Chronic autoimmune thyroiditis (Hashimoto's thyroiditis) in patients with MALT lymphoma.
2008;19:1336-1339 [PMID:18334510 DOI:10.1093/annonc/mdn049]
45 Umehara S,Higashi H,Ohnishi N,Asaka M,Hatakeyama M.Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes,the origin of MALT lymphoma.
2003;22:8337-8342 [PMID:14614457 DOI:10.1038/sj.onc.1207028]
46 Kuo SH,Wu MS,Yeh KH,Lin CW,Hsu PN,Chen LT,Cheng AL.Novel Insights of Lymphomagenesis of
-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma.
2019;11 [PMID:30999581 DOI:10.3390/cancers11040547]
47 Lin WC,Tsai HF,Kuo SH,Wu MS,Lin CW,Hsu PI,Cheng AL,Hsu PN.Translocation of Helicobacter pylori CagA into Human B lymphocytes,the origin of mucosa-associated lymphoid tissue lymphoma.
2010;70:5740-5748 [PMID:20587516 DOI:10.1158/0008-5472.CAN-09-4690]
48 Slomiany BL,Slomiany A.Syk:a new target for attenuation of Helicobacter pylori-induced gastric mucosal inflammatory responses.
2019;27:203-211 [PMID:30820719 DOI:10.1007/s10787-019-00577-6]
49 Slomiany BL,Slomiany A.Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H.pylori:modulatory effect of ghrelin.
2017;25:415-429[PMID:28516374 DOI:10.1007/s10787-017-0360-1]
50 Clark EA,Ledbetter JA.How B and T cells talk to each other.
1994;367:425-428 [PMID:8107800 DOI:10.1038/367425a0]
51 Nakamura H,Kawakami A,Tominaga M,Migita K,Kawabe Y,Nakamura T,Eguchi K.Expression of CD40/CD40 Ligand and Bcl-2 family proteins in labial salivary glands of patients with Sj?gren's syndrome.
1999;79:261-269 [PMID:10092062]
52 Blosse A,Peru S,Levy M,Marteyn B,Floch P,Sifré E,Giese A,Prochazkova-Carlotti M,Azzi Martin L,Dubus P,Mégraud F,Ruskone Fournestraux A,Fabiani B,Copie Bergman C,Robe C,Hahne M,Huard B,Lehours P.APRIL-producing eosinophils are involved in gastric MALT lymphomagenesis induced by Helicobacter sp infection.
2020;10:14858 [PMID:32908188 DOI:10.1038/s41598-020-71792-3]
53 Raderer M,Kiesewetter B.What you always wanted to know about gastric MALT-lymphoma:a focus on recent developments.
2021;13:17588359211033825 [PMID:34621332 DOI:10.1177/17588359211033825]
54 Stergiou IE,Poulaki A,Voulgarelis M.Pathogenetic Mechanisms Implicated in Sj?gren's Syndrome Lymphomagenesis:A Review of the Literature.
2020;9 [PMID:33255258 DOI:10.3390/jcm9123794]
55 Elgueta R,Benson MJ,de Vries VC,Wasiuk A,Guo Y,Noelle RJ.Molecular mechanism and function of CD40/CD40L engagement in the immune system.
2009;229:152-172[PMID:19426221 DOI:10.1111/j.1600-065X.2009.00782.x]
56 Bossen C,Schneider P.BAFF,APRIL and their receptors:structure,function and signaling.
2006;18:263-275 [PMID:16914324 DOI:10.1016/j.smim.2006.04.006]
57 Craig VJ,Cogliatti SB,Arnold I,Gerke C,Balandat JE,Wündisch T,Müller A.B-cell receptor signaling and CD40 Ligand-independent T cell help cooperate in Helicobacter-induced MALT lymphomagenesis.
2010;24:1186-1196 [PMID:20428202 DOI:10.1038/leu.2010.76]
58 García M,Bellosillo B,Sánchez-González B,García-Payarols F,Seoane A,Ferrer AM,Gimeno E,Barranco LE,Torner A,Solé F,Besses C,Serrano S,Salar A.Study of regulatory T-cells in patients with gastric malt lymphoma:influence on treatment response and outcome.
2012;7:e51681 [PMID:23284739 DOI:10.1371/journal.pone.0051681]
59 Curiel TJ.Regulatory T cells and treatment of cancer.
2008;20:241-246[PMID:18508251 DOI:10.1016/j.coi.2008.04.008]
60 Togashi Y,Shitara K,Nishikawa H.Regulatory T cells in cancer immunosuppression-implications for anticancer therapy.
2019;16:356-371 [PMID:30705439 DOI:10.1038/s41571-019-0175-7]
61 D'Elios MM,Amedei A,Manghetti M,Costa F,Baldari CT,Quazi AS,Telford JL,Romagnani S,Del Prete G.Impaired T-cell regulation of B-cell growth in Helicobacter pylori--related gastric lowgrade MALT lymphoma.
1999;117:1105-1112 [PMID:10535873 DOI:10.1016/S0016-5085(99)70395-1]
62 Bergman MP,D'Elios MM.Cytotoxic T cells in H.pylori-related gastric autoimmunity and gastric lymphoma.
2010;2010:104918 [PMID:20617132 DOI:10.1155/2010/104918]
63 Thieblemont C,Bertoni F,Copie-Bergman C,Ferreri AJ,Ponzoni M.Chronic inflammation and extra-nodal marginal-zone lymphomas of MALT-type.
2014;24:33-42 [PMID:24333758 DOI:10.1016/j.semcancer.2013.11.005]
64 Neumeister P,Hoefler G,Beham-Schmid C,Schmidt H,Apfelbeck U,Schaider H,Linkesch W,Sill H.Deletion analysis of the p16 tumor suppressor gene in gastrointestinal mucosa-associated lymphoid tissue lymphomas.
1997;112:1871-1875 [PMID:9178679 DOI:10.1053/gast.1997.v112.pm9178679]
65 Liang R,Chan WP,Kwong YL,Xu WS,Srivastava G,Ho FC.High incidence of BCL-6 gene rearrangement in diffuse large B-cell lymphoma of primary gastric origin.
1997;97:114-118 [PMID:9283593 DOI:10.1016/S0165-4608(96)00388-3]
66 Hussell T,Isaacson PG,Crabtree JE,Spencer J.Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue.
1996;178:122-127 [PMID:8683376 DOI:10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D]
67 Zucca E,Arcaini L,Buske C,Johnson PW,Ponzoni M,Raderer M,Ricardi U,Salar A,Stamatopoulos K,Thieblemont C,Wotherspoon A,Ladetto M;ESMO Guidelines Committee.Marginal zone lymphomas:ESMO Clinical Practice Guidelines for diagnosis,treatment and followup.
2020;31:17-29 [PMID:31912792 DOI:10.1016/j.annonc.2019.10.010]
68 Kotla V,Goel S,Nischal S,Heuck C,Vivek K,Das B,Verma A.Mechanism of action of lenalidomide in hematological malignancies.
2009;2:36 [PMID:19674465 DOI:10.1186/1756-8722-2-36]
69 Quach H,Ritchie D,Stewart AK,Neeson P,Harrison S,Smyth MJ,Prince HM.Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma.
2010;24:22-32 [PMID:19907437 DOI:10.1038/leu.2009.236]
70 Leonard JP,Trneny M,Izutsu K,Fowler NH,Hong X,Zhu J,Zhang H,Offner F,Scheliga A,Nowakowski GS,Pinto A,Re F,Fogliatto LM,Scheinberg P,Flinn IW,Moreira C,Cabe?adas J,Liu D,Kalambakas S,Fustier P,Wu C,Gribben JG;AUGMENT Trial Investigators.AUGMENT:A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma.
2019;37:1188-1199 [PMID:30897038 DOI:10.1200/JCO.19.00010]
71 Kiesewetter B,Troch M,Mayerhoefer ME,Dolak W,Simonitsch-Klupp I,Raderer M.Delayed Efficacy After Treatment With Lenalidomide or Thalidomide in Patients With Mucosa-Associated Lymphoid Tissue Lymphoma.
2016;21:72-75 [PMID:26621040 DOI:10.1634/theoncologist.2015-0176]
72 Bagheri N,Azadegan-Dehkordi F,Rahimian G,Rafieian-Kopaei M,Shirzad H.Role of Regulatory T-cells in Different Clinical Expressions of Helicobacter pylori Infection.
2016;47:245-254 [PMID:27664483 DOI:10.1016/j.arcmed.2016.07.013]
73 Laur AM,Floch P,Chambonnier L,Benejat L,Korolik V,Giese A,Dubus P,Mégraud F,Bandeira A,Lehours P.Regulatory T cells may participate in Helicobacter pylori persistence in gastric MALT lymphoma:lessons from an animal model.
2016;7:3394-3402 [PMID:26657504 DOI:10.18632/oncotarget.6492]
74 Iwaya Y,Kobayashi M,Momose M,Hiraoka N,Sakai Y,Akamatsu T,Tanaka E,Ohtani H,Fukuda M,Nakayama J.High levels of FOXP3
regulatory T cells in gastric MALT lymphoma predict responsiveness to Helicobacter pylori eradication.
2013;18:356-362 [PMID:23551894 DOI:10.1111/hel.12051]
75 Podhorecka M,Goracy A,Szymczyk A,Kowal M,Ibanez B,Jankowska-Lecka O,Macheta A,Nowaczynska A,Drab-Urbanek E,Chocholska S,Jawniak D,Hus M.Changes in T-cell subpopulations and cytokine network during early period of ibrutinib therapy in chronic lymphocytic leukemia patients:the significant decrease in T regulatory cells number.
2017;8:34661-34669 [PMID:28416773 DOI:10.18632/oncotarget.16148]
76 Liu F,Karube K,Kato H,Arita K,Yoshida N,Yamamoto K,Tsuzuki S,Kim W,Ko YH,Seto M.Mutation analysis of NF-κB signal pathway-related genes in ocular MALT lymphoma.
2012;5:436-441 [PMID:22808296]
77 McAllister-Lucas LM,Inohara N,Lucas PC,Ruland J,Benito A,Li Q,Chen S,Chen FF,Yamaoka S,Verma IM,Mak TW,Nú?ez G.Bimp1,a MAGUK family member linking protein kinase C activation to Bcl10-mediated NF-kappaB induction.
2001;276:30589-30597 [PMID:11387339 DOI:10.1074/jbc.M103824200]
78 Hozak RR,Manji GA,Friesen PD.The BIR motifs mediate dominant interference and oligomerization of inhibitor of apoptosis Op-IAP.
2000;20:1877-1885 [PMID:10669762 DOI:10.1128/mcb.20.5.1877-1885.2000]
79 Panwalkar A,Verstovsek S,Giles F.Nuclear factor-kappaB modulation as a therapeutic approach in hematologic malignancies.
2004;100:1578-1589 [PMID:15073843 DOI:10.1002/cncr.20182]
80 Thounaojam MC,Dudimah DF,Pellom ST Jr,Uzhachenko RV,Carbone DP,Dikov MM,Shanker A.Bortezomib enhances expression of effector molecules in anti-tumor CD8+ T lymphocytes by promoting Notch-nuclear factor-κB crosstalk.
2015;6:32439-32455 [PMID:26431276 DOI:10.18632/oncotarget.5857]
81 Pellom ST Jr,Dudimah DF,Thounaojam MC,Uzhachenko RV,Singhal A,Richmond A,Shanker A.Bortezomib augments lymphocyte stimulatory cytokine signaling in the tumor microenvironment to sustain CD8+T cell antitumor function.
2017;8:8604-8621 [PMID:28052005 DOI:10.18632/oncotarget.14365]
82 Guadagnoli M,Kimberley FC,Phan U,Cameron K,Vink PM,Rodermond H,Eldering E,Kater AP,van Eenennaam H,Medema JP.Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas.
2011;117:6856-6865 [PMID:21543761 DOI:10.1182/blood-2011-01-330852]
83 Zimmermann P,Ziesenitz VC,Curtis N,Ritz N.The Immunomodulatory Effects of Macrolides-A Systematic Review of the Underlying Mechanisms.
2018;9:302 [PMID:29593707 DOI:10.3389/fimmu.2018.00302]
84 Ghosh N,Tucker N,Zahurak M,Wozney J,Borrello I,Huff CA.Clarithromycin overcomes resistance to lenalidomide and dexamethasone in multiple myeloma.
2014;89:E116-E120 [PMID:24723438 DOI:10.1002/ajh.23733]
85 Van Nuffel AM,Sukhatme V,Pantziarka P,Meheus L,Sukhatme VP,Bouche G.Repurposing Drugs in Oncology (ReDO)-clarithromycin as an anti-cancer agent.
2015;9:513[PMID:25729426 DOI:10.3332/ecancer.2015.513]
86 Li L,Sun R,Miao Y,Tran T,Adams L,Roscoe N,Xu B,Manyam GC,Tan X,Zhang H,Xiao M,Tzankov A,Visco C,Dybkaer K,Bhagat G,Tam W,Hsi ED,van Krieken JH,You H,Huh J,Ponzoni M,Ferreri AJM,M?ller MB,Piris MA,Zhang M,Winter JN,Medeiros LJ,Rassidakis GZ,Vaupel CA,Li Y,Dakappagari N,Xu-Monette ZY,Young KH.PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma having T-cell infiltration:a study from the International DLBCL Consortium Program.
2019;32:741-754 [PMID:30666052 DOI:10.1038/s41379-018-0193-5]
87 Ferreri AJ,Sassone M,Kiesewetter B,Govi S,Scarfò L,Donadoni G,Raderer M.High-dose clarithromycin is an active monotherapy for patients with relapsed/refractory extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT):the HD-K phase II trial.
2015;26:1760-1765 [PMID:25935794 DOI:10.1093/annonc/mdv214]
88 Ferreri AJM,Cecchetti C,Kiesewetter B,Sassone M,Calimeri T,Perrone S,Ponzoni M,Raderer M.Clarithromycin as a "repurposing drug" against MALT lymphoma.
2018;182:913-915[PMID:28771670 DOI:10.1111/bjh.14878]
89 Govi S,Dognini GP,Licata G,Crocchiolo R,Resti AG,Ponzoni M,Ferreri AJ.Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas:final results of a single-centre phase II trial.
2010;150:226-229 [PMID:20433679 DOI:10.1111/j.1365-2141.2010.08179.x]
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
