Radiofrequency ablation in the management of primary hepatic and biliary tumors
INTRODUCTION
Most primary hepatic tumors are found to be either Hepatocellular Carcinoma (HCC)or Cholangiocarcinoma (CCA).Specifically,within the United States,80%-90% of these tumors are found to be HCCs,and the remaining 10%-15% being CCAs[1-9].These hepatic tumors have a high mortality rate,particularly CCA,which portends a worse prognosis[7,10-14].Traditionally,surgical resection has been shown to have good outcomes in appropriately selected patients.However,with the advent of novel ablative treatment options such as radiofrequency ablation (RFA),the prognosis of both hepatic and biliary tumors can be improved[12,15,16].
Radiofrequency ablation (RFA) is aimed to generate an area of necrosis within the targeted tissue by applying thermal therapy
an electrode[17-20],with a goal to eradicate the tumor while preserving surrounding healthy tissue[12,21,22].Thermal ablation has been used for management of a wide range of lesions,from renal tumors to uterine fibroids.However,more data is emerging in its role as a curative or palliative option in those with primary and secondary hepatobiliary malignancies[11,18-20].In this mini-review article,we discuss the role of RFA in patients with primaryhepatic and biliary tumors.
RFA TECHNIQUE AND PROCEDURE
RFA utilizes electrodes to provide an alternating current,causing the ions to reverberate rapidly,thereby increasing tissue temperature[8,16,23-26].This thermal energy induces coagulative necrosis and subsequent death of the malignant cells.RFA can be accomplished through multiple approaches,including surgical,percutaneous,and more recently,endoscopic modality[19,27,28].Several studies have explored the safety,efficacy and feasibility of RFA for loco-regional control of tumor growth.To facilitate this,a specialized catheter named Endo Luminal RadiofrequencyAblation(ELRA) was developed (STARmed,Goyang,Korea),which is a 7-Fr bipolar catheter with a 1750 mm length,with an automatic temperature probe,allowing the user to avoid excessive heating and collateral damage to surrounding healthy tissue,thus decreasing the rates of procedure-related adverse events[29-32].Four different exposure lengths are available (11,18,22 and 33 mm) to allow RFA of strictures of varying lengths,with recommended power setting of 7-10 W and target temperature of 80 °C for up to 2 min.A similar Habib EndoHPB catheter (Boston Scientific,Marlborough,MA,United States) is an 1800 mm long 8-Fr device with two distal tip electrodes placed 8 mm apart,to achieve biliary RFA.Novel devices have been developed to achieve the same
endoscopic ultrasound (EUS) approach.For example,the Habib endoscopic ultrasound (EUS) RFA device is a 1-Fr wire monopolar electrode inserted inside a standard EUS fine-needle aspiration (FNA) needle that can achieve coagulation of specific target tissue[12,21,33,34].During endoscopic retrograde cholangiography (ERCP),after biliary cannulation a guidewire is passed through the strictured segment of bile duct,over which the RFA catheter is advanced and electrodes are positioned under fluoroscopic guidance to achieve ablation over bursts of 60 s.For longer strictures,stepwise ablation is performed to cover the entire length,or alternately catheter with varying exposure length can be utilized,if available.This modality can also be used to treat tumor/tissue ingrowth within metallic stents placed for cholangiocarcinoma (Figure 1).For strictures involving the hilum,ablation of both right and left hepatic ducts is performed after placement of two bilateral guidewires.After improvement of stricture,upstream debris removal is performed,followed by cholangiogram to assess for complications including bile leak,prior to stent placement.
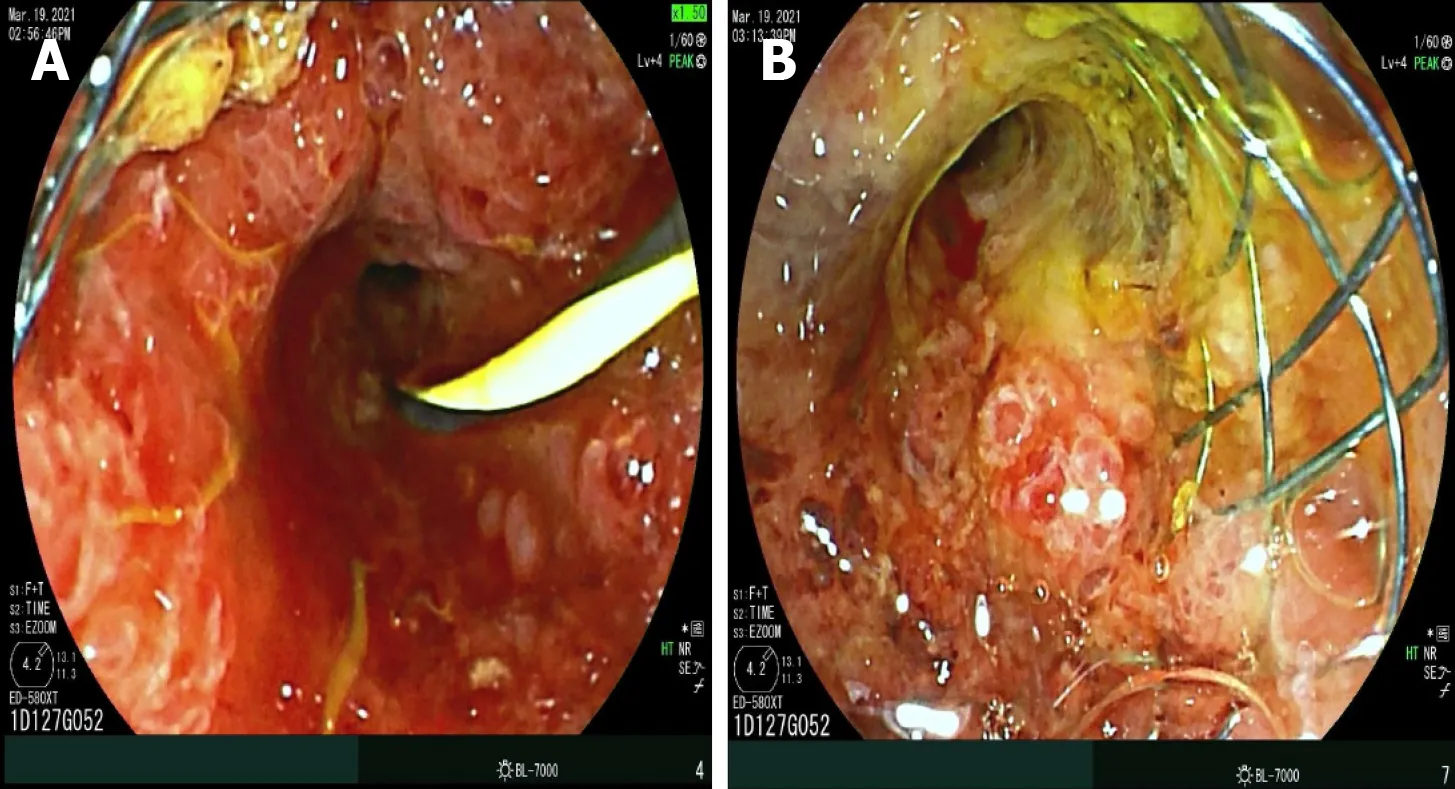
RFA IN CCC
CCA represents approximately 2%-3% of malignancies arising from the gastrointestinal (GI) system,but is second most common primary liver tumor[7,34,35].Specifically,these malignancies arise from the cells that line the biliary tree,and categorized as extra-hepatic or intra-hepatic,depending on their extent of ductal infiltration and location in relation to the cystic duct insertion;as most famously reported using the Bismuth-Corlette system[13,34].Supplementary classifications of CCAs have been proposed,which in addition to tumor extent within the biliary system also take into account the size of the tumor,vascular (hepatic artery/portal vein) and lymph node involvement,distant metastases,and estimated post-resection hepatic volume[36],which have advantage over Bismuth-Corlette system which does not provide information on vascular encasement or metastatic disease,includes only peri-hilar CCA but not intrahepatic CCA,and does not necessarily determine local resectability,and hence of limited prognostic value.In fact,there is emerging evidence that although resection of type IV CCA is technically demanding with high morbidity,it can be performed with low mortality and offers better survival probability in selected patients[37].
Who knows what may not happen before that? thought the miller s daughter; and besides, she saw no other way out of it, so she promised the manikin what he demanded, and he set to work once more and spun the straw into gold
To summarize,RFA is a successful strategy for loco-regional management of extrahepatic CCA,management of malignant biliary obstruction,as well as blocked metallic stents.The performance of RFA is operator dependent,and not protocol based,and hence timing and interval of RFA remains unclear,as well as choice of stents (plastic
metallic).For intra-hepatic CCA,the access using ERCP-RFA catheters can be challenging,and alternative approaches may include EUS-RFA or percutaneous RFA by Interventional Radiology.Alternatives to RFA include Photodynamic Therapy (PDT),Microwave Ablation (MWA) and Irreversible Electroporation Ablation (IRE),all of which are complex and expensive procedures,which require highly specialized equipment,have side effects (photosensitivity with PDT)and complication profile,and hence not commonly performed worldwide.IRE is a non-thermal ablation modality,the basic principle of which is to create irreversible pores in cellular bi-lipid membranes by subjecting them to series of high intensity electrical pulses for short duration of time,resulting in cell death due to apoptosis,especially used for tumors located close to porta-hepatis.On the other hand,in MWA,tumor tissue is destroyed by direct hyperthermic injury produced by electromagnetic waves emitted from non-insulated portions of antenna,resulting in larger volume of active heating resulting in shorter procedure times,higher tissue temperatures beyond the threshold of water vaporization and less susceptibility to the heat sink effect of blood flow.Detailed discussion regarding these modalities is beyond the scope of this mini-review manuscript.
HCC is the most common primary liver cancer and has the third-highest cancerrelated mortality worldwide,exceeding 700000 deaths per year[2,3,5,6,45-47].There are different causes of HCC,which vary worldwide;in Africa,aflatoxin B1 and chronic Hepatitis B infection seem to account for the most incidence of HCC,whereas cases in North America,Japan,and Europe are related to alcoholism and Hepatitis C infection[2,3,5,6,45-47].Currently,the curative management options for HCC include liver transplantation,hepatectomy,or ablative therapies.Most patients diagnosed with HCC are not surgical candidates due to the advanced tumor size,invasion,or presence of metastasis[1-4,48].Most management algorithms worldwide employ a specific scoring system named Barcelona Clinic Liver Cancer guidelines[1-6,48-50],to aids clinicians in determining the most appropriate management modality.Patients with early-stage HCC without any vascular invasion are classified as BCLC-A,which are suitable candidates for resection,ablation,or transplantation.On the other end of the treatment spectrum,patients with extra-hepatic tumor spread or vascular invasion are classified as BCLC-C,and are best managed with systemic therapies such as Sorafenib[1-6,48-50].
Several studies have explored the role of combination therapy with RFA.In a recent meta-analysis,the pooled results showed that the 1-,3-,5-year overall survival rate in the combined RFA+hepatectomy group were comparable with those in the hepatectomy alone group (OR=0.77,0.96,0.88;
0.33,0.88,0.70,respectively).Similarly,there was no significant difference in 1-,3-,5-year disease free survival rate between the combined group and the surgical alone group (OR=0.57,0.83,0.72;
0.17,0.37,0.32,respectively).These results indicated that the hepatectomy combined with RFA could reach a long-term survival outcome similar to curative surgical resection for multifocal HCC patients,and this approach may be a promising alternative for patients with marginal liver function or complicated tumor distribution[75].But,RFA+hepatectomy is limited due to its increased rate of post-op complications such as liver failure and death.Transcatheter arterial chemoembolization(TACE) is another commonly used percutaneous non-ablative treatment for HCC[76],which when combined with RFA yields a feasible treatment strategy with promising outcomes.In study by Kim
[77],1 mo,6 mo,and 1-year tumor responses of TACERFA were similar to those of RFA and better than those of TACE.A distinct advantage of this combination therapy may be in patients with tumors located close to major vessels,wherein TACE occludes the hepatic artery flow,allowing a larger area for RFA ablation.This strategy minimizes the “heat-sink” effect associated with RFA.Regardless,the TACE-RFA group showed longer hospital stay and more frequent patient discomfort requiring medication than TACE or RFA monotherapy groups (
0.001),as well as the frequency of overall complications after TACE-RFA was higher than TACE (
0.006) or RFA (
0.009)[52,74,76-78].Finally,RFA is also being utilized in combination with Sorafenib for management of HCC.A recent metaanalysis (15 studies,2227 patients) showed that compared to RFA-alone,the patients in RFA+Sorafenib had longer 1-,2-and 3-year overall survival (
0.05),better overall efficacy (
0.0001),longer RFA interval (
0.001) and lower 2-year recurrence rate (
0.02).However,this came at the cost of higher adverse reactions compared to RFAalone group,including hand-foot skin reactions (
0.001),diarrhea and constipation (
0.0001),hypertension (
0.009) and alopecia (
0.001)[79].Therefore,cognizance of overall adverse events is necessary while choosing the most optimal strategy.Despite these limitations,overall improvements in technology under development show promising prospects in the treatment of HCC.
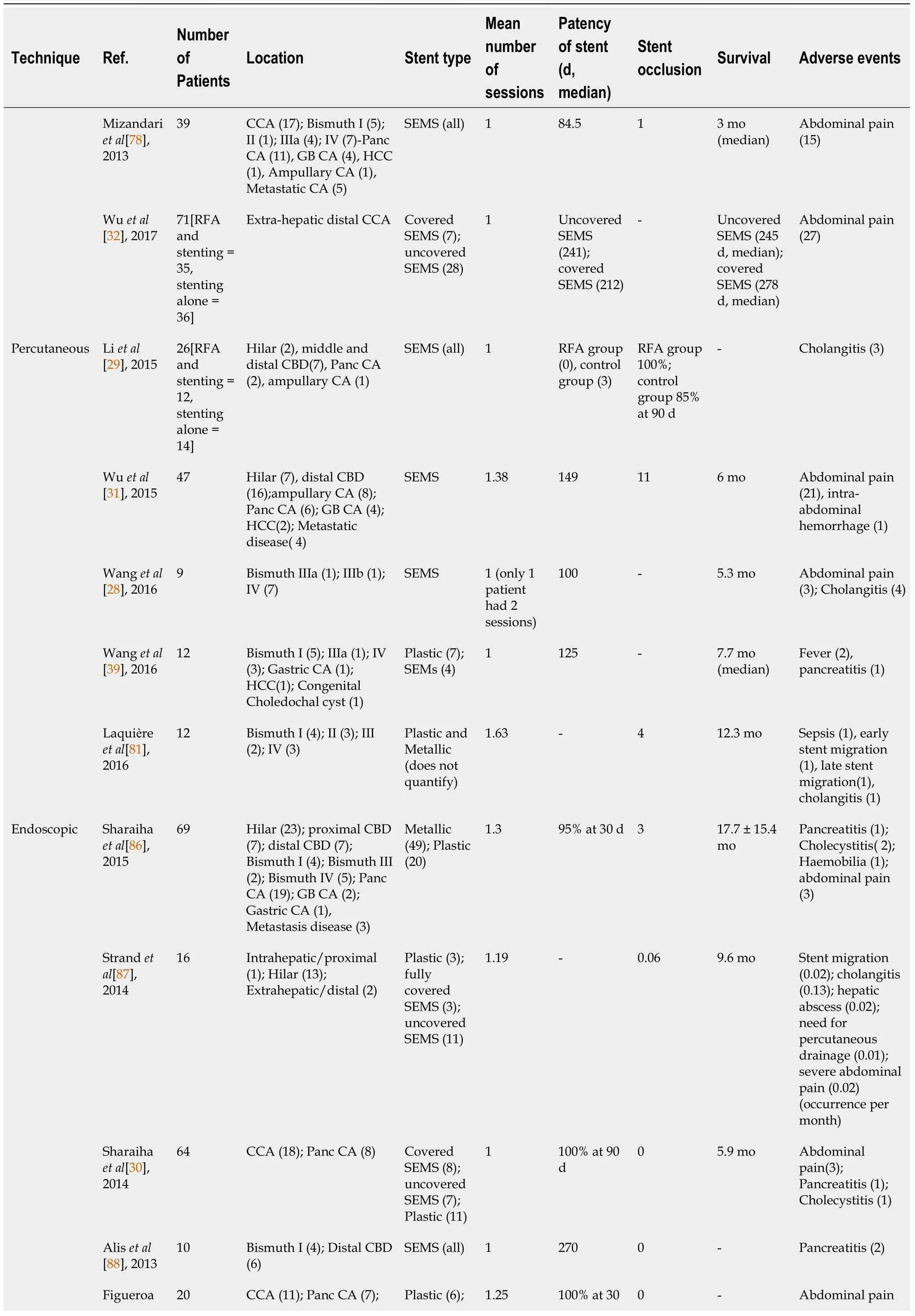
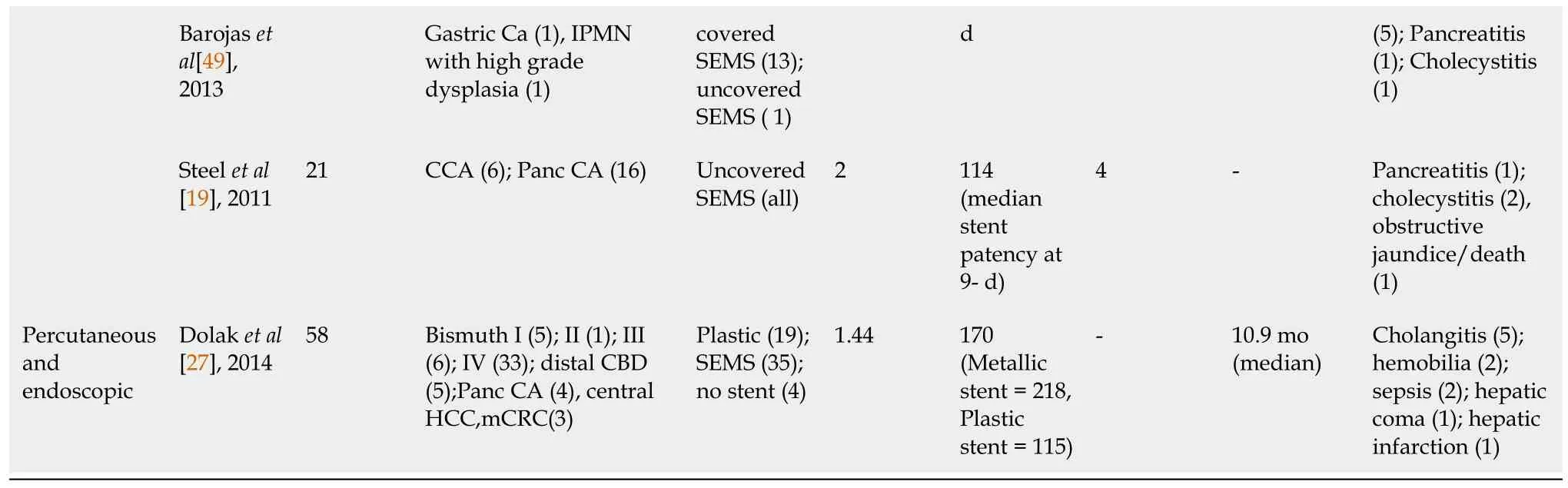
When it comes to the treatment of CCAs,anatomical location and resectability play a crucial role.For those lesions that are considered resectable,surgical resection can be curable.Chemotherapy and radiation are typically utilized for unresectable lesions or can be used as neoadjuvant therapy for resectable tumors.For those tumors that cause obstruction,biliary drainage is usually the mainstay therapy with stent placement[29,32,38].At present,extra-hepatic CCA is considered the condition most effectively treated with biliary RFA.Performance of RFA for intrahepatic CCA is challenging,and can be achieved
ERCP or EUS or percutaneous approach.RFA has also been employed to prolong the patency of stents in malignant obstructive tumors[27,38,39].Typically the deployment of a self-expandable metallic stent (SEMS) is the mainstay palliative therapy in these patients.By prolonging the patency of stents,it improves survival and quality of life in patients with unresectable CCA.
RFA IN HCC
Multiple studies have appraised the efficacy of RFA in the treatment of CCA and stent patency[33,40,41].Cui and colleagues evaluated the effect of RFA on stent patency in malignant biliary obstruction,and while there was no significant difference in the overall survival,patency time was significantly increased in the RFA group at 7.6 mo when compared to 4.3 mo in the stent without RFA group.Another retrospective study by Li
[29] determined hat stent patency was prolonged in those patients who underwent RFA plus stenting compared to stenting alone (81%
35%)with a
<0.05.Furthermore,a meta-analysis by Sofi and colleagues,which included eight observational studies and one randomized controlled trial of RFA in malignant biliary obstruction showed not only a significantly prolonged stent patency in the RFA group when compared to the control group,but also a significant increase in overall survival in the RFA group (
=504;95%CI:1.145-1.7;
0.01)[18].Yang
[20]performed a randomized control trial on patients with unresectable distal CCA and perihilar CCA;one group received RFA plus stenting (
=32) and the other group received stenting alone (
=33).Compared to stenting alone,the RFA plus stent group had a statistically significant increase in both patency (6.8 mo;95%CI:3.6-8.2
3.4 mo;95%CI:2.4-6.5) and overall survival (13.2 mo
8.3 mo)[20].These results are in contrast to previous reports,like by Wu
[32],which has shown efficacy of RFA for stent patency,but no survival benefit.A detailed summary is provided in Table 1.Few studies have also compared Photodynamic therapy (PDT) with RFA,mostly without any statistically significant difference in overall survival between the two treatment approaches[42].However,one of the retrospective studies did show that RFA conferred a short-term advantage in decline in bilirubin[43,44].
Now it s just me and my hard driven guiltBehind a wall of emptiness I allowed to be built.I m trapped in my body, just wanting to runBack to my youth with its laughter and fun. But the chase1 is over and there s no place to hideEverything is gone, including my pride.With reality suddenly right in my faceI m scared, alone and stuck in this place.
RFA is the most widely used thermal ablative procedure used in patients with HCC[49,54,57,58],the success of which is inversely related to tumor size.Complete remission is achieved in approximately 90%-92% in those with tumor size<2 cm whereas remission rates decrease to 20%-40% in those ≥ 2 cm in size[59].While theoretically,multipolar electrodes may expand the ablation zone of RFA,this has not panned out in clinical studies.Cartier
[60] compared traditional monopolar electrode RFA with multipolar electrodes in patients with tumors>2.5 cm,and found no difference in residual tumor or recurrence.RFA seems to be a safe treatment option with procedure-related mortality of approximately 0.2% and an overall complication rate of 2.2%[61,62].A novel RFA technique being studied is the "no-touch RFA protocol," which involves inserting multiple electrodes within the tissue that surrounds the tumor[62],which avoids direct contact with the tumor,allowing thermal ablation to be conducted with decreased risk of tumor seeding by the probe.
With the introduction of the Milan criteria,an increase in liver transplantation has been witnessed,ushering in a new era of curative treatment for HCC[51,52].However,transplantation is dependent on donor availability,and since there are a limited number of donors,only a finite number of patients can undergo successful treatment.More importantly,patients may spend long periods of time awaiting transplant,allowing cancer to progress,which may disqualify formerly eligible patients from transplantation.To avoid this clinical dilemma,ablative techniques such as RFA become important for the crucial role they can play in delaying the malignancy progression[24,53-56].A distinct advantage of these ablation techniques is that they can be performed safely on suboptimal surgical candidates.
Several studies have also explored the comparative success rates of RFA
hepatectomy in HCC.A meta-analysis by Xu
[72] indicated that RFA and surgical hepatectomy had similar overall survival at 1 year (relative risk [RR],1.39;95%confidence interval [CI]:0.36,5.33;
0.63) and 3 years (RR,1.40;95%CI:0.75,2.62;
0.29),whereas RFA resulted in decreased overall survival compared with HR at 5 years (RR:1.91;95%CI:1.32,2.79;
0.001)[72].However,closer analysis of subgroup data,results showed no difference in survival between the groups in tumors less than 2.0 cm in size[72].The Surveillance,Epidemiology and End Results (SEER) database explored the same question further stratified by age[65],and noted that patients older than 65 years with tumors less than 2 cm had similar survival to their propensitymatched group age less than 65 years.Interestingly,those<65 years and tumors>3.0 cm had an increased overall survival with hepatectomy compared to RFA.However,large-scale studies have not been able to incorporate the novel RFA techniques previously discussed compared to hepatectomy[59,73,74].Further studies will need to be conducted to answer this question.
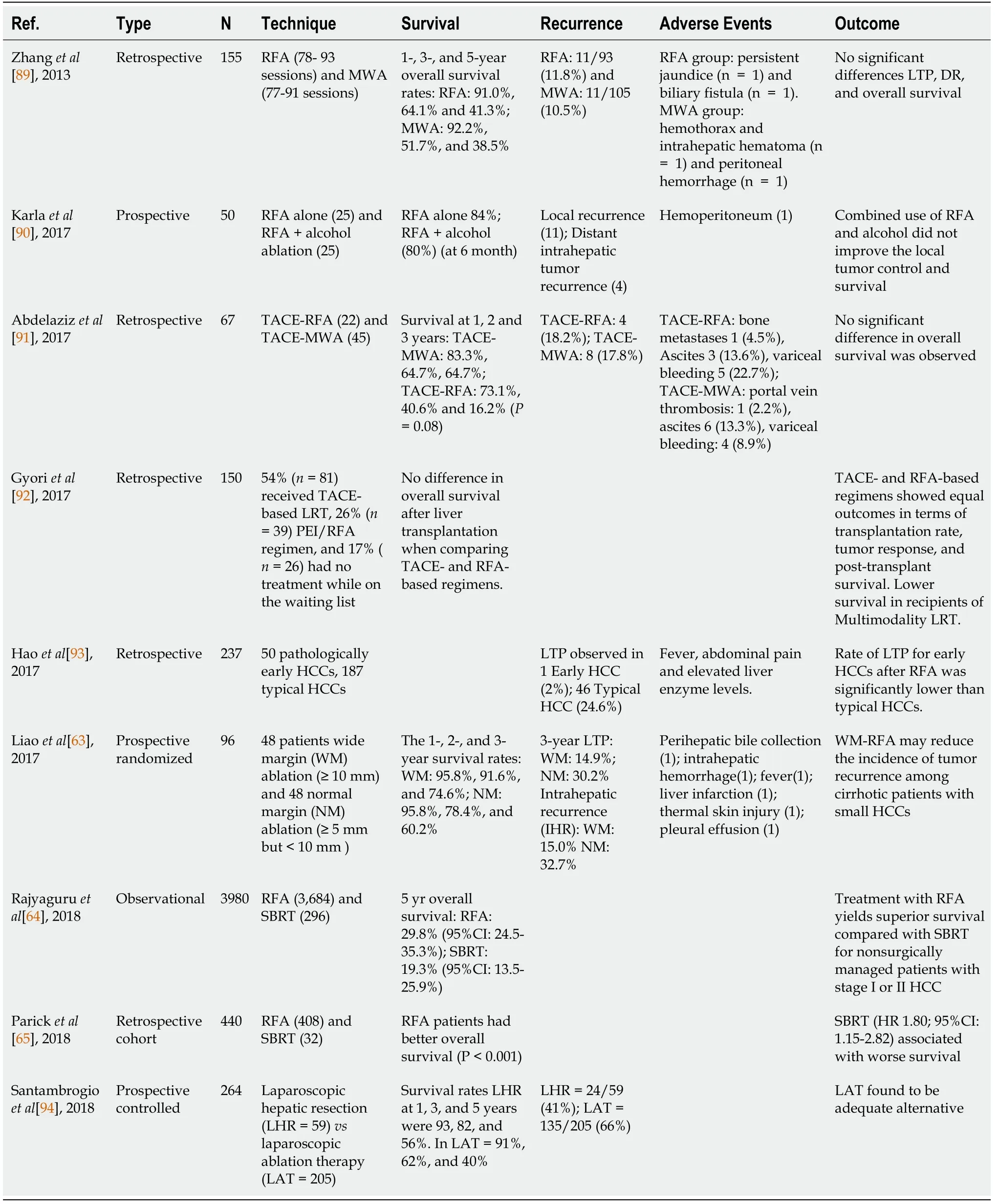
Several studies have investigated the effectiveness of RFA in HCC (Table 2).Liao
[63] randomized 96 patients into those undergoing wide margin (WM ≥ 10 mm)ablation (
=48) and normal margin (NM:≥ 5 but<10 mm) ablation (
=48),and followed for mean period of 38.3 ± 4.8 mos.When analyzed based on intention-to-treat strategy,the 3-year incidences of local tumor progression (LTP) (14.9%
30.2%),intrahepatic recurrence (IHR) (15.0%
32.7%),and recurrence-free survival (RFS)(31.7 ± 12.1
24.0 ± 11.7 mo) for WM group were significantly improved compared to NM group[63].Getting recurrence-free survival advantage with RFA is a major success,for which RFA is adopted widely worldwide for smaller HCC,especially in non-resectable candidates.In regards to the “no-touch RFA protocol," a multicenter retrospective study of HCC<5 cm in diameter (
=362) showed effectiveness of this approach over monopolar RFA in terms of recurrence rates[62,63],but no statistical difference in 5-year survival rates (monopolar 37.2%
no-touch multipolar 46.4%
0.378).Some investigators have proposed that stereotactic body radiotherapy (SBRT)was more effective than RFA,which has been challenged in recent studies[64,65].In 2018,Rajyaguru
[64] compared the effectiveness of RFA (
=3684) against SBRT (
=296),and their analysis support superior survival with RFA for non-surgically managed patients with stage I or II HCC.Various studies have investigated predictive factors to achieve improved outcomes in HCC when utilizing RFA.In a recent metaanalysis by Giardini
[61,65-68] (34 studies;
=11,216),alpha-fetoprotein (AFP)<20 ng/mL,Child-Pugh class A and albumin-bilirubin index of 1 were noted to confer increased survival benefit.In addition,survival also increased in patients with single tumor<2 cm in diameter and preserved hepatic function[61,69-71].
He recognized them in his turn and greeted them joyfully147, but when they turned afterwards to look for the Rosy Mole, the Chaffinch, and the Trotting-Mouse, they had vanished, and in their places stood a lovely lady whom they did not know, the Black Bird, and the Green Giant
The wedding was fixed16, and the maiden17 had already arrived; but because of her great ugliness, however, she shut herself in her room, and allowed no one to see her, and Maid Maleen had to take her her meals from the kitchen
ADVERSE EVENTS AND LIMITATIONS OF RFA
Several adverse events have been associated with RFA,the most common being postprocedure mild abdominal pain following either endoscopic or percutaneous RFA approaches.There seems to be a higher incidence of bleeding with percutaneous RFA,whereas a higher association of pancreatitis with the endoscopic approach[59,80].Other post-procedure complications,such as hemobilia and hepatic artery pseudoaneurysms,have been postulated to be due to thermal injury[38,81].This can be avoided with the newer ELRA RF catheter,which has a temperature probe.Further complications have been listed in Tables 1 and 2.
RFA does have its limitations.The therapeutic efficacy of RFA is inversely associated with tumor size and location[59].RFA needs direct contact with the tissue,which can pose a challenge to treat tumors in inaccessible sites.Furthermore,tumors in close proximity to large vessels pose interesting therapeutic challenges[10,18,22,41,82,83].Tumors located near large portal and hepatic vein branches can result in a"heat-sink" effect,which results in the inability to reach maximal ablation temperatures,thereby causing incomplete cell death[84].It is important to keep in mind that RFA cannot be used in pregnancy or patients with cardiac devices or coagulopathy[24,73,82,83,85].
The next day the two sisters were at the ball, and so was Cinderella, but dressed more magnificently than before. The King s son was always by her, and never ceased his compliments and kind speeches to her; to whom all this was so far from being tiresome40 that she quite forgot55 what her godmother had recommended to her; so that she, at last, counted the clock striking twelve when she took it to be no more than eleven; she then rose up and fled, as nimble as a deer.56 The Prince followed, but could not overtake her. She left behind one of her glass slippers, which the Prince took up most carefully. She got home but quite out of breath, and in her nasty old clothes, having nothing left her of all her finery but one of the little slippers, fellow to that she dropped. The guards at the palace gate were asked if they had not seen a princess go out.
CONCLUSION
RFA has been established as novel and safe minimally invasive management tool for HCC.While multiple studies optimizing these techniques have shown promising results in patients with CCA,the low incidence of these biliary tumors makes it challenging to coordinate high-powered RCTs comparing various techniques and treatment strategies.It is paramount that future studies are coordinated through collaboration between various institutions of excellence for the progress of this still novel technique.
1 Avolio AW,Cillo U,Salizzoni M,De Carlis L,Colledan M,Gerunda GE,Mazzaferro V,Tisone G,Romagnoli R,Caccamo L,Rossi M,Vitale A,Cucchetti A,Lupo L,Gruttadauria S,Nicolotti N,Burra P,Gasbarrini A,Agnes S;Donor-to-Recipient Italian Liver Transplant (D2R-ILTx) Study Group.Balancing donor and recipient risk factors in liver transplantation:the value of D-MELD with particular reference to HCV recipients.
2011;11:2724-2736 [PMID:21920017 DOI:10.1111/j.1600-6143.2011.03732.x]
2 Bosetti C,Levi F,Boffetta P,Lucchini F,Negri E,La Vecchia C.Trends in mortality from hepatocellular carcinoma in Europe,1980-2004.
2008;48:137-145 [PMID:18537177 DOI:10.1002/hep.22312]
3 Bruix J,Gores GJ,Mazzaferro V.Hepatocellular carcinoma:clinical frontiers and perspectives.
2014;63:844-855 [PMID:24531850 DOI:10.1136/gutjnl-2013-306627]
4 Bruix J,Sherman M;Practice Guidelines Committee,American Association for the Study of Liver Diseases.Management of hepatocellular carcinoma.
2005;42:1208-1236 [PMID:16250051 DOI:10.1002/hep.20933]
5 Bruix J,Sherman M;American Association for the Study of Liver Diseases.Management of hepatocellular carcinoma:an update.
2011;53:1020-1022 [PMID:21374666 DOI:10.1002/hep.24199]
6 Forner A,Llovet JM,Bruix J.Hepatocellular carcinoma.
2012;379:1245-1255 [PMID:22353262 DOI:10.1016/s0140-6736(11)61347-0]
7 Green BL,House MG.Nonsurgical Approaches to Treat Biliary Tract and Liver Tumors.
2019;28:573-586 [PMID:31472906 DOI:10.1016/j.soc.2019.06.013]
8 Lencioni R,Cioni D,Crocetti L,Franchini C,Pina CD,Lera J,Bartolozzi C.Early-stage hepatocellular carcinoma in patients with cirrhosis:long-term results of percutaneous image-guided radiofrequency ablation.
2005;234:961-967 [PMID:15665226 DOI:10.1148/radiol.2343040350]
9 Loriaud A,Denys A,Seror O,Vietti Violi N,Digklia A,Duran R,Trillaud H,Hocquelet A.Hepatocellular carcinoma abutting large vessels:comparison of four percutaneous ablation systems.
2018;34:1171-1178 [PMID:29457510 DOI:10.1080/02656736.2018.1440017]
10 Buerlein RCD,Wang AY.Endoscopic Retrograde Cholangiopancreatography-Guided Ablation for Cholangiocarcinoma.
2019;29:351-367 [PMID:30846158 DOI:10.1016/j.giec.2018.11.006]
11 Chahal P,Baron TH.Endoscopic palliation of cholangiocarcinoma.
2006;22:551-560 [PMID:16891889 DOI:10.1097/01.mog.0000239872.12081.a4]
12 Labib PL,Davidson BR,Sharma RA,Pereira SP.Locoregional therapies in cholangiocarcinoma.
2017;4:99-109 [PMID:29367874 DOI:10.2217/hep-2017-0014]
13 Qureshi K,Jesudoss R,Al-Osaimi AM.The treatment of cholangiocarcinoma:a hepatologist's perspective.
2014;16:412 [PMID:25183579 DOI:10.1007/s11894-014-0412-2]
14 Razumilava N,Gores GJ.Classification,diagnosis,and management of cholangiocarcinoma.
2013;11:13-21.e1;quiz e3 [PMID:22982100 DOI:10.1016/j.cgh.2012.09.009]
15 Nault JC,Sutter O,Nahon P,Ganne-Carrié N,Séror O.Percutaneous treatment of hepatocellular carcinoma:State of the art and innovations.
2018;68:783-797 [PMID:29031662 DOI:10.1016/j.jhep.2017.10.004]
16 Shiina S,Tateishi R,Arano T,Uchino K,Enooku K,Nakagawa H,Asaoka Y,Sato T,Masuzaki R,Kondo Y,Goto T,Yoshida H,Omata M,Koike K.Radiofrequency ablation for hepatocellular carcinoma:10-year outcome and prognostic factors.
2012;107:569-77;quiz 578[PMID:22158026 DOI:10.1038/ajg.2011.425]
17 Lee EW,Chen C,Prieto VE,Dry SM,Loh CT,Kee ST.Advanced hepatic ablation technique for creating complete cell death:irreversible electroporation.
2010;255:426-433 [PMID:20413755 DOI:10.1148/radiol.10090337]
18 Sofi AA,Khan MA,Das A,Sachdev M,Khuder S,Nawras A,Lee W.Radiofrequency ablation combined with biliary stent placement
stent placement alone for malignant biliary strictures:a systematic review and meta-analysis.
2018;87:944-951.e1 [PMID:29108980 DOI:10.1016/j.gie.2017.10.029]
19 Steel AW,Postgate AJ,Khorsandi S,Nicholls J,Jiao L,Vlavianos P,Habib N,Westaby D.Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction.
2011;73:149-153 [PMID:21184881 DOI:10.1016/j.gie.2010.09.031]
20 Yang J,Wang J,Zhou H,Zhou Y,Wang Y,Jin H,Lou Q,Zhang X.Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma:a randomized trial.
2018;50:751-760 [PMID:29342492 DOI:10.1055/s-0043-124870]
21 Larghi A,Rimba? M,Tringali A,Bo?koski I,Rizzatti G,Costamagna G.Endoscopic radiofrequency biliary ablation treatment:A comprehensive review.
2019;31:245-255 [PMID:30444547 DOI:10.1111/den.13298]
22 Lee DH,Lee JM.Recent Advances in the Image-Guided Tumor Ablation of Liver Malignancies:Radiofrequency Ablation with Multiple Electrodes,Real-Time Multimodality Fusion Imaging,and New Energy Sources.
2018;19:545-559 [PMID:29962861 DOI:10.3348/kjr.2018.19.4.545]
23 Kim YS,Lim HK,Rhim H,Lee MW,Choi D,Lee WJ,Paik SW,Koh KC,Lee JH,Choi MS,Gwak GY,Yoo BC.Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma:analysis of prognostic factors.
2013;58:89-97 [PMID:23023009 DOI:10.1016/j.jhep.2012.09.020]
24 Livraghi T,Meloni F,Di Stasi M,Rolle E,Solbiati L,Tinelli C,Rossi S.Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis:Is resection still the treatment of choice?
2008;47:82-89 [PMID:18008357 DOI:10.1002/hep.21933]
25 Tateishi R,Shiina S,Teratani T,Obi S,Sato S,Koike Y,Fujishima T,Yoshida H,Kawabe T,Omata M.Percutaneous radiofrequency ablation for hepatocellular carcinoma.An analysis of 1000 cases.
2005;103:1201-1209 [PMID:15690326 DOI:10.1002/cncr.20892]
26 Yan K,Chen MH,Yang W,Wang YB,Gao W,Hao CY,Xing BC,Huang XF.Radiofrequency ablation of hepatocellular carcinoma:long-term outcome and prognostic factors.
2008;67:336-347 [PMID:17765421 DOI:10.1016/j.ejrad.2007.07.007]
27 Dolak W,Schreiber F,Schwaighofer H,Gschwantler M,Plieschnegger W,Ziachehabi A,Mayer A,Kramer L,Kopecky A,Schrutka-K?lbl C,Wolkersd?rfer G,Madl C,Berr F,Trauner M,Püsp?k A;Austrian Biliary RFA Study Group.Endoscopic radiofrequency ablation for malignant biliary obstruction:a nationwide retrospective study of 84 consecutive applications.
2014;28:854-860 [PMID:24196547 DOI:10.1007/s00464-013-3232-9]
28 Wang F,Li Q,Zhang X,Jiang G,Ge X,Yu H,Nie J,Ji G,Miao L.Endoscopic radiofrequency ablation for malignant biliary strictures.
2016;11:2484-2488 [PMID:27284336 DOI:10.3892/etm.2016.3235]
29 Li TF,Huang GH,Li Z,Hao CF,Ren JZ,Duan XH,Zhang K,Chen C,Han XW,Jiao DC,Zhang MF,Wang YL.Percutaneous transhepatic cholangiography and intraductal radiofrequency ablation combined with biliary stent placement for malignant biliary obstruction.
2015;26:715-721 [PMID:25817458 DOI:10.1016/j.jvir.2015.01.037]
30 Sharaiha RZ,Natov N,Glockenberg KS,Widmer J,Gaidhane M,Kahaleh M.Comparison of metal stenting with radiofrequency ablation
stenting alone for treating malignant biliary strictures:is there an added benefit?
2014;59:3099-3102 [PMID:25033929 DOI:10.1007/s10620-014-3264-6]
31 Wu TT,Li HC,Li WM,Ao GK,Lin H,Zheng F,Song JY.Percutaneous Intraluminal Radiofrequency Ablation for Malignant Extrahepatic Biliary Obstruction:A Safe and Feasible Method.
2015;60:2158-2163 [PMID:25648642 DOI:10.1007/s10620-015-3547-6]
32 Wu TT,Li WM,Li HC,Ao GK,Zheng F,Lin H.Percutaneous Intraductal Radiofrequency Ablation for Extrahepatic Distal Cholangiocarcinoma:A Method for Prolonging Stent Patency and Achieving Better Functional Status and Quality of Life.
2017;40:260-269 [PMID:27743089 DOI:10.1007/s00270-016-1483-2]
33 Cui W,Fan W,Lu M,Zhang Y,Yao W,Li J,Wang Y.The safety and efficacy of percutaneous intraductal radiofrequency ablation in unresectable malignant biliary obstruction:A single-institution experience.
2017;17:288 [PMID:28438130 DOI:10.1186/s12885-017-3278-5]
34 Shaib Y,El-Serag HB.The epidemiology of cholangiocarcinoma.
2004;24:115-125 [PMID:15192785 DOI:10.1055/s-2004-828889]
35 Ferlay J,Shin HR,Bray F,Forman D,Mathers C,Parkin DM.Estimates of worldwide burden of cancer in 2008:GLOBOCAN 2008.
2010;127:2893-2917 [PMID:21351269 DOI:10.1002/ijc.25516]
36 Deoliveira ML,Schulick RD,Nimura Y,Rosen C,Gores G,Neuhaus P,Clavien PA.New staging system and a registry for perihilar cholangiocarcinoma.
2011;53:1363-1371 [PMID:21480336 DOI:10.1002/hep.24227]
37 Ebata T,Mizuno T,Yokoyama Y,Igami T,Sugawara G,Nagino M.Surgical resection for Bismuth type IV perihilar cholangiocarcinoma.
2018;105:829-838 [PMID:28488733 DOI:10.1002/bjs.10556]
38 Tal AO,Vermehren J,Friedrich-Rust M,Bojunga J,Sarrazin C,Zeuzem S,Trojan J,Albert JG.Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction.
2014;6:13-19 [PMID:24527176 DOI:10.4253/wjge.v6.i1.13]
39 Wang Y,Cui W,Fan W,Zhang Y,Yao W,Huang K,Li J.Percutaneous intraductal radiofrequency ablation in the management of unresectable Bismuth types III and IV hilar cholangiocarcinoma.
2016;7:53911-53920 [PMID:27322076 DOI:10.18632/oncotarget.10116]
40 Cui W,Wang Y,Fan W,Lu M,Zhang Y,Yao W,Li J.Comparison of intraluminal radiofrequency ablation and stents
stents alone in the management of malignant biliary obstruction.
2017;33:853-861 [PMID:28540797 DOI:10.1080/02656736.2017.1309580]
41 Lee YN,Jeong S,Choi HJ,Cho JH,Cheon YK,Park SW,Kim YS,Lee DH,Moon JH.The safety of newly developed automatic temperature-controlled endobiliary radiofrequency ablation system for malignant biliary strictures:A prospective multicenter study.
2019;34:1454-1459 [PMID:30861593 DOI:10.1111/jgh.14657]
42 Yang J,Shen H,Jin H,Lou Q,Zhang X.Treatment of unresectable extrahepatic cholangiocarcinoma using hematoporphyrin photodynamic therapy:A prospective study.
2016;16:110-118 [PMID:27720942 DOI:10.1016/j.pdpdt.2016.10.001]
43 Patel J,Rizk N,Kahaleh M.Role of photodynamic therapy and intraductal radiofrequency ablation in cholangiocarcinoma.
2015;29:309-318 [PMID:25966430 DOI:10.1016/j.bpg.2015.02.008]
44 Schmidt A,Bloechinger M,Weber A,Siveke J,von Delius S,Prinz C,Schmitt W,Schmid RM,Neu B.Short-term effects and adverse events of endoscopically applied radiofrequency ablation appear to be comparable with photodynamic therapy in hilar cholangiocarcinoma.
2016;4:570-579 [PMID:27536367 DOI:10.1177/2050640615621235]
45 Ikenaga N,Chijiiwa K,Otani K,Ohuchida J,Uchiyama S,Kondo K.Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion.
2009;13:492-497 [PMID:19011945 DOI:10.1007/s11605-008-0751-0]
46 Mazzaferro V,Regalia E,Doci R,Andreola S,Pulvirenti A,Bozzetti F,Montalto F,Ammatuna M,Morabito A,Gennari L.Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis.
1996;334:693-699 [PMID:8594428 DOI:10.1056/nejm199603143341104]
47 Murray KF,Carithers RL Jr;AASLD.AASLD practice guidelines:Evaluation of the patient for liver transplantation.
2005;41:1407-1432 [PMID:15880505 DOI:10.1002/hep.20704]
48 Arii S,Teramoto K,Kawamura T,Okamoto H,Kaido T,Mori A,Imamura M.Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience.
2001;8:397-403 [PMID:11702247 DOI:10.1007/s005340100000]
49 Figueroa-Barojas P,Bakhru MR,Habib NA,Ellen K,Millman J,Jamal-Kabani A,Gaidhane M,Kahaleh M.Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer:a novel palliation technique.
2013;2013:910897 [PMID:23690775 DOI:10.1155/2013/910897]
50 Freeman RB,Edwards EB,Harper AM.Waiting list removal rates among patients with chronic and malignant liver diseases.
2006;6:1416-1421 [PMID:16686765 DOI:10.1111/j.1600-6143.2006.01321.x]
51 Huang J,Yan L,Cheng Z,Wu H,Du L,Wang J,Xu Y,Zeng Y.A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria.
2010;252:903-912 [PMID:21107100 DOI:10.1097/SLA.0b013e3181efc656]
52 Liu W,Zheng Y,He W,Zou R,Qiu J,Shen J,Yang Z,Zhang Y,Wang C,Wang Y,Zuo D,Li B,Yuan Y.Microwave
radiofrequency ablation for hepatocellular carcinoma within the Milan criteria:a propensity score analysis.
2018;48:671-681 [PMID:30063081 DOI:10.1111/apt.14929]
53 DuBay DA,Sandroussi C,Kachura JR,Ho CS,Beecroft JR,Vollmer CM,Ghanekar A,Guba M,Cattral MS,McGilvray ID,Grant DR,Greig PD.Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation.
2011;13:24-32 [PMID:21159100 DOI:10.1111/j.1477-2574.2010.00228.x]
54 Fisher RA,Maluf D,Cotterell AH,Stravitz T,Wolfe L,Luketic V,Sterling R,Shiffman M,Posner M.Non-resective ablation therapy for hepatocellular carcinoma:effectiveness measured by intentionto-treat and dropout from liver transplant waiting list.
2004;18:502-512 [PMID:15344951 DOI:10.1111/j.1399-0012.2004.00196.x]
55 Lu DS,Yu NC,Raman SS,Lassman C,Tong MJ,Britten C,Durazo F,Saab S,Han S,Finn R,Hiatt JR,Busuttil RW.Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation.
2005;41:1130-1137 [PMID:15841454 DOI:10.1002/hep.20688]
56 Toso C,Dupuis-Lozeron E,Majno P,Berney T,Kneteman NM,Perneger T,Morel P,Mentha G,Combescure C.A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list.
2012;56:149-156 [PMID:22271250 DOI:10.1002/hep.25603]
57 Fontana RJ,Hamidullah H,Nghiem H,Greenson JK,Hussain H,Marrero J,Rudich S,McClure LA,Arenas J.Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma:a safe and effective bridge to liver transplantation.
2002;8:1165-1174 [PMID:12474157 DOI:10.1053/jlts.2002.36394]
58 Hansen PD,Cassera MA,Wolf RF.Ablative technologies for hepatocellular,cholangiocarcinoma,and metastatic colorectal cancer of the liver.
2015;24:97-119 [PMID:25444471 DOI:10.1016/j.soc.2014.09.003]
59 Jiang YQ,Wang ZX,Deng YN,Yang Y,Wang GY,Chen GH.Efficacy of Hepatic Resection
Radiofrequency Ablation for Patients With Very-Early-Stage or Early-Stage Hepatocellular Carcinoma:A Population-Based Study With Stratification by Age and Tumor Size.
2019;9:113 [PMID:30863723 DOI:10.3389/fonc.2019.00113]
60 Cartier V,Boursier J,Lebigot J,Oberti F,Fouchard-Hubert I,Aubé C.Radiofrequency ablation of hepatocellular carcinoma:Mono or multipolar?
2016;31:654-660 [PMID:26414644 DOI:10.1111/jgh.13179]
61 Casadei Gardini A,Marisi G,Canale M,Foschi FG,Donati G,Ercolani G,Valgiusti M,Passardi A,Frassineti GL,Scarpi E.Radiofrequency ablation of hepatocellular carcinoma:a meta-analysis of overall survival and recurrence-free survival.
2018;11:6555-6567 [PMID:30323628 DOI:10.2147/ott.S170836]
62 Hocquelet A,Aubé C,Rode A,Cartier V,Sutter O,Manichon AF,Boursier J,N'kontchou G,Merle P,Blanc JF,Trillaud H,Seror O.Comparison of no-touch multi-bipolar
monopolar radiofrequency ablation for small HCC.
2017;66:67-74 [PMID:27422750 DOI:10.1016/j.jhep.2016.07.010]
63 Liao M,Zhong X,Zhang J,Liu Y,Zhu Z,Wu H,Zeng Y,Huang J.Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis:A prospective randomized trial.
2017;115:971-979 [PMID:28334430 DOI:10.1002/jso.24607]
64 Rajyaguru DJ,Borgert AJ,Smith AL,Thomes RM,Conway PD,Halfdanarson TR,Truty MJ,Kurup AN,Go RS.Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients:Analysis of the National Cancer Database.
2018;36:600-608 [PMID:29328861 DOI:10.1200/jco.2017.75.3228]
65 Parikh ND,Marshall VD,Green M,Lawrence TS,Razumilava N,Owen D,Singal AG,Feng M.Effectiveness and cost of radiofrequency ablation and stereotactic body radiotherapy for treatment of early-stage hepatocellular carcinoma:An analysis of SEER-medicare.
2018;62:673-681 [PMID:29877615 DOI:10.1111/1754-9485.12754]
66 Nicoli N,Casaril A,Marchiori L,Mangiante G,Hasheminia AR.Treatment of recurrent hepatocellular carcinoma by radiofrequency thermal ablation.
2001;8:417-421 [PMID:11702250 DOI:10.1007/s005340100003]
67 Pai M,Spalding D,Jiao L,Habib N.Use of bipolar radiofrequency in parenchymal transection of the liver,pancreas and kidney.
2012;29:43-47 [PMID:22441619 DOI:10.1159/000335732]
68 Pulvirenti A,Garbagnati F,Regalia E,Coppa J,Marchiano A,Romito R,Schiavo M,Fabbri A,Burgoa L,Mazzaferro V.Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation.
2001;33:1516-1517 [PMID:11267402 DOI:10.1016/s0041-1345(00)02577-x]
69 Brillet PY,Paradis V,Brancatelli G,Rangheard AS,Consigny Y,Plessier A,Durand F,Belghiti J,Sommacale D,Vilgrain V.Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation:a prospective study with histopathologic comparison.
2006;186:S296-S305 [PMID:16632691 DOI:10.2214/ajr.04.1927]
70 Chan AC,Poon RT,Cheung TT,Chok KS,Chan SC,Fan ST,Lo CM.Survival analysis of reresection
radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma.
2012;36:151-156 [PMID:22030561 DOI:10.1007/s00268-011-1323-0]
71 Cho YK,Kim JK,Kim MY,Rhim H,Han JK.Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies.
2009;49:453-459[PMID:19065676 DOI:10.1002/hep.22648]
72 Xu XL,Liu XD,Liang M,Luo BM.Radiofrequency Ablation
Hepatic Resection for Small Hepatocellular Carcinoma:Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis.
2018;287:461-472 [PMID:29135366 DOI:10.1148/radiol.2017162756]
73 Majumdar A,Roccarina D,Thorburn D,Davidson BR,Tsochatzis E,Gurusamy KS.Management of people with early-or very early-stage hepatocellular carcinoma:an attempted network meta-analysis.
2017;3:CD011650 [PMID:28351116 DOI:10.1002/14651858.CD011650.pub2]
74 Mohkam K,Dumont PN,Manichon AF,Jouvet JC,Boussel L,Merle P,Ducerf C,Lesurtel M,Rode A,Mabrut JY.No-touch multibipolar radiofrequency ablation
surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm.
2018;68:1172-1180 [PMID:29410287 DOI:10.1016/j.jhep.2018.01.014]
75 Xu LL,Zhang M,Yi PS,Zheng XB,Feng L,Lan C,Tang JW,Ren SS,Xu MQ.Hepatic resection combined with radiofrequency ablation
hepatic resection alone for multifocal hepatocellular carcinomas:A meta-analysis.
2017;37:974-980 [PMID:29270762 DOI:10.1007/s11596-017-1836-3]
76 Martin AN,Wilkins LR,Das D,Johnston LE,Bauer TW,Adams RB,Zaydfudim VM.Efficacy of Radiofrequency Ablation
Transarterial Chemoembolization for Patients with Solitary Hepatocellular Carcinoma ≤ 3 cm.
2019;85:150-155 [PMID:30819290]
77 Kim W,Cho SK,Shin SW,Hyun D,Lee MW,Rhim H.Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma:comparison with TACE or RFA monotherapy.
2019;44:2283-2292 [PMID:30806742 DOI:10.1007/s00261-019-01952-1]
78 Mizandari M,Pai M,Xi F,Valek V,Tomas A,Quaretti P,Golfieri R,Mosconi C,Guokun A,Kyriakides C,Dickinson R,Nicholls J,Habib N.Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction:feasibility and early results.
2013;36:814-819 [PMID:23232859 DOI:10.1007/s00270-012-0529-3]
79 Jin M,Yu Q,Liu Y,Xu W,Fu X,Ji B.Safety and Efficacy of Physical Thermal Ablation Combined Sorafenib for Hepatocellular Carcinoma:A Meta-analysis.
2021;9:149-159[PMID:34007796 DOI:10.14218/jcth.2020.00125]
80 Germani G,Pleguezuelo M,Gurusamy K,Meyer T,Isgrò G,Burroughs AK.Clinical outcomes of radiofrequency ablation,percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma:a meta-analysis.
2010;52:380-388 [PMID:20149473 DOI:10.1016/j.jhep.2009.12.004]
81 Laquière A,Boustière C,Leblanc S,Penaranda G,Désilets E,Prat F.Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma.
2016;30:1242-1248 [PMID:26162420 DOI:10.1007/s00464-015-4322-7]
82 Kang TW,Lim HK,Cha DI.Percutaneous ablation for perivascular hepatocellular carcinoma:Refining the current status based on emerging evidence and future perspectives.
2018;24:5331-5337 [PMID:30598578 DOI:10.3748/wjg.v24.i47.5331]
83 Lurje I,Czigany Z,Bednarsch J,Roderburg C,Isfort P,Neumann UP,Lurje G.Treatment Strategies for Hepatocellular Carcinoma-a Multidisciplinary Approach.
2019;20 [PMID:30909504 DOI:10.3390/ijms20061465]
84 Weber JC,Navarra G,Jiao LR,Nicholls JP,Jensen SL,Habib NA.New technique for liver resection using heat coagulative necrosis.
2002;236:560-563 [PMID:12409660 DOI:10.1097/00000658-200211000-00004]
85 Koda M,Ueki M,Maeda Y,Mimura KI,Okamoto K,Matsunaga Y,Kawakami M,Hosho K,Murawaki Y.The influence on liver parenchymal function and complications of radiofrequency ablation or the combination with transcatheter arterial embolization for hepatocellular carcinoma.
2004;29:18-23 [PMID:15135342 DOI:10.1016/j.hepres.2004.02.001]
86 Sharaiha RZ,Sethi A,Weaver KR,Gonda TA,Shah RJ,Fukami N,Kedia P,Kumta NA,Clavo CM,Saunders MD,Cerecedo-Rodriguez J,Barojas PF,Widmer JL,Gaidhane M,Brugge WR,Kahaleh M.Impact of Radiofrequency Ablation on Malignant Biliary Strictures:Results of a Collaborative Registry.
2015;60:2164-2169 [PMID:25701319 DOI:10.1007/s10620-015-3558-3]
87 Strand DS,Cosgrove ND,Patrie JT,Cox DG,Bauer TW,Adams RB,Mann JA,Sauer BG,Shami VM,Wang AY.ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma.
2014;80:794-804 [PMID:24836747 DOI:10.1016/j.gie.2014.02.1030]
88 Alis H,Sengoz C,Gonenc M,Kalayci MU,Kocatas A.Endobiliary radiofrequency ablation for malignant biliary obstruction.
2013;12:423-427 [PMID:23924501 DOI:10.1016/s1499-3872(13)60066-1]
89 Zhang L,Wang N,Shen Q,Cheng W,Qian GJ.Therapeutic efficacy of percutaneous radiofrequency ablation
microwave ablation for hepatocellular carcinoma.
2013;8:e76119 [PMID:24146824 DOI:10.1371/journal.pone.0076119]
90 Kalra N,Kang M,Duseja AK,Bhatia A,Singh V,Dhiman RK,Rajwanshi A,Chawla YK,Khandelwal N.Comparison of radiofrequency ablation alone and in combination with percutaneous ethanol injection for management of hepatocellular carcinoma.
2017;146:S30-S37[PMID:29578192 DOI:10.4103/ijmr.IJMR_1812_15]
91 Abdelaziz AO,Abdelmaksoud AH,Nabeel MM,Shousha HI,Cordie AA,Mahmoud ShH,Medhat E,Omran D,Elbaz TM.Transarterial Chemoembolization Combined with Either Radiofrequency or Microwave Ablation in Management of Hepatocellular Carcinoma Asian Pac J Cancer Prev 2017;18:189-194 [PMID:28240516 DOI:10.22034/apjcp.2017.18.1.189]
92 Gy?ri GP,Felsenreich DM,Silberhumer GR,Soliman T,Berlakovich GA.Multimodality locoregional treatment strategies for bridging HCC patients before liver transplantation.
2017;49:236-243 [PMID:29104589 DOI:10.1007/s10353-017-0487-8]
93 Hao Y,Numata K,Ishii T,Fukuda H,Maeda S,Nakano M,Tanaka K.Rate of local tumor progression following radiofrequency ablation of pathologically early hepatocellular carcinoma.
2017;23:3111-3121 [PMID:28533668 DOI:10.3748/wjg.v23.i17.3111]
94 Santambrogio R,Barabino M,Bruno S,Mariani N,Maroni N,Bertolini E,Franceschelli G,Opocher E.Surgical Resection
Ablative Therapies Through a Laparoscopic Approach for Hepatocellular Carcinoma:a Comparative Study.
2018;22:650-660 [PMID:29235004 DOI:10.1007/s11605-017-3648-y]
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
