Early-onset colorectal cancer:Current insights and future directions
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer deaths worldwide.The International Agency for Research on Cancer estimated that there were 1.93 million new cases of CRC and 935000 deaths from CRC in 2020[1].Early-onset CRC (EOCRC),largely defined as CRC occurring in adults younger than 50 years old,has seen an alarming rising trend in recent years[2-5].
“She looks so funny now,” said Randi, and it was true. Once while playing beauty shop, Kelly had cut her own blonde hair along with Chatty Baby’s, giving them both a raggedy crew cut. Kelly’s hair had eventually grown back, but Chatty Baby’s never had. Now the wisps of blonde hair that stuck out all over the dolls head made her look a little lost and forgotten. But her eyes were still bright blue and she still had a smile on her face, even though her face was smudged here and there by the touch of many chubby20 little fingers.
A recent systemic review of 40 studies spanning 12 countries across five continents has found a nearly 30% increase in incidence of EOCRC around the world over the past 20 years,largely driven by increasing incidence in the United States,Australia,and Canada[6].Since 1994,the incidence of EOCRC has been increasing by around 2%per year.This is alarming given that the overall incidence of and death from CRC has been on the decline[2].An observational study done on CRC incidence in the United States population according to the Surveillance,Epidemiology,and End Results(SEER) registries found a steep increase in EOCRC incidence from age 49-50 years,with 92.9% of cases being invasive lesions picked up on screening[7].This likely reflects that a significant proportion of the populations were screened too late,given that the goal of screening was to remove premalignant lesions to prevent malignant transformation.In 2018,the American Cancer Society (ACS) lowered their recommended age for average-risk adults to start screening at 45 years old[8].Although this method allows early detection of advanced adenomas or CRC to reduce disease burden and mortality,this mass screening approach will likely lead to a substantial increase in cost and burden to the healthcare system.
The little old woman had heard in her sleep the great, rough, gruff voice of the Great, Huge Bear; but she was so fast asleep that it was no more to her than the roaring of wind or the rumbling9 of thunder
EOCRC tends to have a predominantly left colonic and rectal distribution,a higher proportion of mucinous and signet ring histologic subtype,poorer cell differentiation,a higher pathologic grade,and a more advanced stage at presentation[9-11].Although hereditary cancer syndromes and family history account for approximately 30% of EOCRC cases,the majority appear to arise sporadically[12].To date,the underlying etiologies of this rising trend have not yet been fully elucidated.Identifying specific risk factors or causes to this trend can allow for the establishment of better risk-stratification models and more targeted screening to tackle this global phenomenon.
Multiple postulated risk factors have been identified that may be driving factors to the development of EOCRC.Exposure to many potential elements from an early age from conception to adulthood may predispose to a higher risk of EOCRC.This includes external factors such as socioeconomic background,lifestyle,diet,and antibiotic exposure;and intrinsic factors,such as genetics,gut microbiota,and oxidative stress[13].
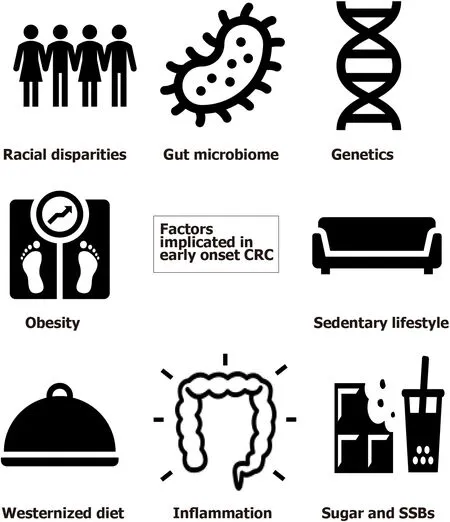
Apart from the well-established risk factors for CRC such as male gender,smoking,alcoholism,family history of CRC,type 2 diabetes,and inflammatory bowel disease,many studies have attempted to study additional demographic and environmental factors that may be specific risk factors for EOCRC[10,14,15].A meta-analysis examining 20 studies through MEDLINE and Embase database search found that Caucasian ethnicity,obesity,and hyperlipidemia,as well as male gender,alcohol,and history of CRC in a first-degree relative,were all significantly associated with the development of EOCRC[16].A more sedentary lifestyle or occupation,ulcerative colitis,hypertension,and diet-related factors were also found to have an association with increased risk in some studies[14].Here we discuss in more detail some of the key suspects implicated in the development of EOCRC (Figure 1).
RACIAL DISPARITIES
Our diet from birth has a role in shaping our gut microbiome.Understanding the relationship between diet and gut dysbiosis teaches us that how we shape our diets at an early age could impact the development of CRC.Thus,it is important to encourage healthy eating habits from childhood to maintain a healthy microbiota.Nevertheless,it remains difficult to prove the causative link between dysbiosis in early human development and its association with EOCRC,and further research in this area is needed.
Recognizing genetic alterations that can predispose to early onset of high-risk adenoma or CRC is crucial for deciding on early screening regimes and therapeutic strategies.Around 28% of EOCRC patients have a positive family history[71].Patients with a first-degree relative of CRC have up to a four-fold increased lifetime risk of CRC[72].Those with a known family history of a high-penetrance hereditary cancer syndrome,such as Lynch syndrome or adenomatous polyposis coli (APC),are at a particularly high risk and require an onset of colonoscopy screening at a much earlier age than the general population[73,74].For non-hereditary cases,according to the ACS guidelines,those with a first-degree relative of CRC diagnosed before age 60 should also start colonoscopy screening from age 40,or 10 years younger than the earliest diagnosed relative[72].However,low adherence to early screening guidelines is one of the major obstacles in EOCRC prevention.A study of 2473 patients with EOCRC found that family history-based early screening criteria were only adhered to in 25% of cases,and nearly all these patients could have had CRC diagnosed earlier or even prevented had they followed these guidelines[75].This highlights the importance of public education on cancer screening programs.
These efforts have led to tangible results with the gap closing between Whites and Blacks[19].In fact,the incidence of rectal cancer in Whites has now surmounted that of the Blacks and Hispanics in recent years,and the overall incidence of EOCRC is now similar in the two groups since 2015[2].Results of a SEER analysis examining the difference in incidence of CRC amongst White and Black EOCRC patients from 1992-1996 to 2010-2014 showed that there was a 47% relative increase in CRC incidence in Whites,compared to a 1% relative increase in Blacks[20].The rise in EOCRC is mainly due to an increase in rectal cancer,which was seen most strikingly in the White population.This suggests that rectal cancer may have its own distinct characteristics and etiological differences from colon cancer.Nevertheless,the incidence of EOCRC is still climbing steadily regardless of ethnicity,highlighting the need for further research into meaningful interventions to curb this rise.
OBESITY AND SEDENTARY LIFESTYLE
The link between diet,nutrients,and the pathogenesis of EOCRC is complex,with a myriad of processes involving immune signaling,genetic predisposition,and alterations in the gut microbiome.Other significant food exposures that may play a role in CRC include dietary additives,nitrate-containing foods,synthetic food colorings,monosodium glutamate,
.[13,54].Further studies on dietary causation links will bring to light any potential preventative measures for EOCRC.
23.Others are there: Neither of the younger sisters has any sense. Instead of questioning or even noticing that the preceding girls are stuck to the geese, the last two sisters reach out to take a feather, even when they are warned.The youngest sister basically exhibits behavior similar to that of Dummling s at the beginning of the tale. The difference is that the youngest girl, like her two sisters, is motivated by greed and just tries to take.Return to place in story.
A recent prospective cohort study,which examined dietary patterns in 29474 women who underwent colonoscopy at<50 years of age,found that a Westernized diet was positively associated with high-risk distal or rectal adenomas,whereas healthier diets such as a prudent diet,Dietary Approaches to Stop Hypertension,Alternative Mediterranean Diet,and Alternative Healthy Eating Index were inversely associated with early onset adenomas[43].Interestingly,some studies have found that the genetic composition of tumors associated with a Western diet tends to be KRAS wild-type,and BRAF-wild type[44].These genetic compositions are consistent with the typical features of EOCRC.
Leading a sedentary lifestyle has also been recognized as an emerging global health problem due to increased desk work,the rising trend of e-commerce,and inactive media consumption since a young age[27].A prospective study examining television viewing time (as a surrogate for sedentary time) in almost 90000 women aged 25 to 42 years in the United States found that more than 1 hour of daily TV viewing was associated with a 12% increased risk of CRC,particularly rectal cancer.More than 2 hours of TV viewing was associated with a 70% increase in risk.The risk appeared even higher in subgroups of patients with a high BMI,physical inactivity,and smokers[28].
Physical inactivity may result in lower energy use,higher caloric intake,and unhealthy dietary intake.It may also correlate with impaired glucose regulation or gut dysbiosis.Some studies have examined the role of increased physical activity to improve gut health by promoting certain bacterial species in the gut microbiome[29-31].All in all,this highlights the importance of physical activity and controlling the obesity pandemic to prevent EOCRC.
WESTERN DIET
A growing adoption of a non-Mediterranean,Western diet worldwide has been consistently shown in the literature to be an important risk factor[32,33].A diet high in red,processed meat,and low in fibre from a young age has been shown to affect the gut microbiota and drive inflammation processes[34-36].Westernized cooking methods,such as deep-frying,grilling,or roasting,generate more advanced glycation end-products (AGEs),which are complex compounds produced from food that is rich in fat and protein[37,38].They are involved in promoting oxidative stress and chronic inflammation,which in turn promote a microenvironment favorable for colorectal carcinogenesis.Many studies have shown that AGEs are responsible for signal pathways involved in colitis-associated colorectal carcinogenesis seen in inflammatory bowel disease[39].Mediterranean food,on the other hand,has low AGE levels and has been found to be protective against the development of CRC[40-42].
Of course,there are multiple confounding variables that may affect the relationship between obesity and EOCRC.This includes a reverse causality effect where CRC may induce weight loss.Obesity itself could also be a surrogate for other known risk factors for CRC.Metabolic syndrome,increased insulin resistance,raised insulin-like growth factor 1 (IGF-1),and raised low-density lipoprotein are all positively correlated with an increased risk for EOCRC[21,24].
A meta-analysis recently published suggested a strong association of higher intake of dietary fibre,calcium,and yoghurt with a reduced risk of CRC,with convincing evidence that intake of a Western diet and processed meat is associated with a higher risk of EOCRC[45].Interestingly,the impact of yoghurt and calcium may be related with the modulation of the gut microbiome,such as the presence of lactic acidproducing bacteria,which may reduce the level of carcinogens in the gut.Yoghurt also creates a lower pH in the colon,which may be more accommodating for probiotics[46].This supports the idea that modulating the gut microbiome with prebiotics and/or probiotics may have a potential role in preventing the development of CRC,which will be further discussed in a later section.
SUGAR
One of the other culprits in the plethora of Western food that may be a culprit for EOCRC is sugar.Refined sugars (including glucose,fructose,sucrose,and maltose) are cheap and widely available worldwide.Sugar consumption in the form of snacks,desserts,sweets,or sugar-sweetened beverages has steeply increased especially during childhood and adolescence.Over the last decade,sugar consumption globally has grown from 154 to 171 million metric tons from 2009/2010 to 2019/2020[47].This climb was found to be most significant in developing or low-income countries[48].In a large United States cohort study that analyzed 95464 female registered nurses’ dietary habits from the Nurses’ Health Study II,it was found that high sugar (especially fructose) intake during adolescence was significantly associated with an increased risk of colorectal adenomas.Consuming two or more,rather than one,sugar-sweetened beverages a day in adolescence further increased the risk of EOCRC by two-fold[49].
Several mechanisms that tie sugar intake to the development of CRC have been postulated.High intake of sugar can promote obesity,insulin resistance,and type 2 diabetes[50,51].Sugar,specifically fructose,may have a direct effect on the gut microbiome,leading to chronic inflammation and a heightened susceptibility of the colorectal epithelium to cellular damage[52].Fructose also produces AGEs,which as previously discussed,has a potentially significant role in carcinogenesis[53].Hyperinsulinemia and elevated IGF-1 levels can stimulate cell proliferation and differentiation,inhibit apoptosis,and in turn enhance tumor development.As adolescence is a period of pronounced physiological changes that include decreased insulin sensitivity and hyperinsulinemia,this stage of development may be particularly susceptible to the effects of a high sugar intake[49].
Obesity has long been associated with an increased risk of CRC[21].According to a recent propensity-weighted analysis which included 133008 adults diagnosed with EOCRC in the United States between 1999 and 2018,there was a strong association between EOCRC and a raised body mass index (BMI) of ≥ 30 kg/m
,along with an earlier age of diabetes diagnosis[22].A meta-analysis in 2017 found a 30% increased risk of CRC in men and a 12% increased risk of CRC in women for every 5 kg/m
increment increase in BMI[23].There is also an increased risk of early-onset advanced adenoma amongst obese patients[24].The underlying mechanism behind the association between obesity and EOCRC is unclear,although it is postulated that there is an interplay between the risk of obesity,estrogen levels,and the risk of CRC,with obesity being a driver of chronic inflammation[25,26].
GUT MICROBIOME
Alterations of gut microbiome composition (or gut dysbiosis) can lead to dysregulation of multiple pathways in the body.Extensive or prolonged antibiotics use can destroy normal gut flora and lead to colonization of unwelcome pathogens.Several microorganisms,such as
,
,
,
,and
,have been discovered to have a role in colon carcinogenesis.These pathogens can promote gut inflammation,produce cancerassociated metabolites,and activate oncogenic signaling pathways[61].Chronic inflammation from bacterial infection or inflammatory bowel disease can cause epithelial barrier dysfunction and weaken host defenses.Different dietary exposures can lead to significant shifts in the gut microbiome,favoring organisms capable of utilizing those specific nutrients.High-fat diets can lead to accumulation of lipopolysaccharides that can promote inflammation and increase VEGF-C expression,which is a key regulator for lymphangiogenesis and lymph node metastasis in CRC[62].One study found that a drastic increase in fibre intake over 2 wk led to a change in microbiome composition to fibre-degrading bacteria,such as
and
,which has been associated with anti-oncogenic properties[63-65].
It is estimated that 100 billion bacteria reside in the gastrointestinal tract (with a large proportion present in the colon),maintaining a symbiotic relationship with the human host[55].The gut microbiota maintains gut homeostasis and functions and is often considered the first line of defense against pathogens.The composition of the gut microbiome is dynamic and subject to change by multiple factors throughout our lives.The first 1-2 years of life are pivotal for the development of the gut microbiota[56].From birth,the microbiota composition is believed to differ significantly depending on the mode of delivery.Vaginally delivered babies tend to have more
,whereas Caesarean-delivered babies tend to have delayed colonization of facultative anaerobes such as
[57].Breast-fed and bottle-fed babies also have markedly different gut microbiota composition,with breastfed babies having a much higher abundance of bacteria that are thought to be beneficial,such as
and
species[58].The composition of the gut microbiota stabilizes in early adulthood,but is still influenced by exposures such as diet,antibiotics,stress,and inflammation
The gut microbiome is responsible for the synthesis of many important vitamins or molecules for our human body,such as butyrate,folate,biotin,and cobalamin[59].Some of these molecules are important in reducing bacterial translocation and promoting anti-inflammatory properties,and are essential in maintaining gut barrier integrity[60].
Probiotics have long been marketed to the general public as a dietary supplement for their potential beneficial effects on the gut[66].The replenishment of beneficial intestinal microbial communities may help stimulate epithelial cell proliferation,reduce pathogenic overgrowth,ameliorate gut inflammation,and potentially reduce the risk of CRC[67-69].Studies have also shown that certain strains of probiotics may be effective as an adjuvant agent to CRC treatment[70].Yet,its effects specifically on CRC treatment are not well studied and further investigation is required.
African Americans have been known to be at higher risk for the development of CRC compared with Caucasians,and this is usually associated with an earlier-onset and worse outcome[17].Potential reasons for this disparity include lower socioeconomic status,limited access to healthcare,and lack of awareness of screening.Steps have been taken over the years to close this gap in CRC risk with the American College of Gastroenterology and American Society of Gastrointestinal Endoscopists guidelines recommending an earlier age to start CRC screening for African Americans[18].
She went to the parsonage, and begged that she might be taken into service there. She would be industrious27, she said, and do everything that she could; she did not mind about the wages as long as she had a roof over her, and was with good people. The pastor28’s wife had pity on her, and took her into service. And she was industrious and thoughtful. She sat quiet and listened when the pastor read aloud from the Bible in the evening. All the children liked her very much, but when they spoke about dress and grandeur29 and beauty she would shake her head.
GENETIC FEATURES
I was to get a pony4, but before we could even start looking, I had to live up to my end of the bargain. I had to try to talk. I had a chart of weekly tasks I had to accomplish. I had to answer the phone five times per week, something I had never done before. I had to make five phone calls to my friends. I had to say one word to my teacher at school and the list went on. For a child with Selective Mutism, saying one word to someone can be like climbing Mount Everest.
Several studies have found that a significant proportion of EOCRC patients carrying a genetic mutation have no family history of CRC[10,71].Apart from the wellrecognized hereditary cancer syndromes accounting for around 13% of EOCRC cases,a wide spectrum of low to moderate penetrance sporadic mutations have recently been found in these patients,including some genes not traditionally associated with CRC[71,76].A genome-wide association study found up to 140 single nucleotide polymorphisms associated with CRC[77].Genetic mutations are much more common in EOCRC compared with those diagnosed at a later age[78] and may have a cumulative effect.However,in the absence of a positive family history,a proportion of these patients will not be enrolled into early screening programs with strategies to identify such patients being an unmet need[79].
The pathogenesis of CRC involves a complex sequence of multistep genetic alterations.There are three main genetic pathways of CRC carcinogenesis:Chromosomal instability (CIN),microsatellite instability (MSI),and CpG island methylator phenotype (CIMP) pathways[80].Each pathway is associated with specific genetic and epigenetic alterations.The CIN pathway is characterized by an accumulation of mutations in the tumor-suppressor and oncogenes,including
,
,and
amongst others,accounting for 85% of sporadic CRC cases.The MSI pathway,on the other hand,is a state of genetic hypermutability due to impaired DNA mismatch repair (MMR).MSI is the hallmark of Lynch syndrome-associated tumors,an autosomal dominant disorder characterized by the presence of DNA
genes (
,
and
),accounting for around 8% of EOCRC cases[71,81].Lynch syndrome increases the lifetime risk of CRC to 52%-82% depending on the pathogenic variant involved[82].The CIMP pathway and BRAF V600E mutation are thought to be the molecular hallmark of the serrated pathway and are usually associated with proximal lesions[83].
EOCRC has distinct genetic features compared with late-onset CRC.A retrospective review of around 36000 CRC patients comparing genetic characteristics in different age groups showed that EOCRC patients are more likely to be MSI and have CTNNB1,ATM mutations,and CIMP hypermethylation.The consensus molecular subtype 1 was the most common CRC subtype in patients younger than 40 years old.There were fewer BRAF V600 mutations (<4%) in patients less than 30 years old.KRAS,NRAS,and BRAF mutations in the mitogen-activated protein kinase pathway were lowest in the 18-29-year-old group (48%),and highest in the 70-year-old or older group (65%-70%)[84].Hypermethylation of
,
,and
genes were also found to be suggestive of earlier diagnosis of CRC[85].
Certain genetic mutations may infer a higher rate of progression or be predictive factors for treatment resistance.KRAS mutation confers resistance to anti-EGFR therapy.Several studies have demonstrated that MSI tumors have a lack of response to 5FU-based chemotherapy[86].Given that around 1 in 5 patients with EOCRC have a germline mutation,broad germline testing should be considered for all EOCRC patients to guide treatment modalities,prognostication,counselling to family members,and chemoprevention strategies[76,87].
The boat drove ashore2 below their father s castle, and both princes were received with open arms by their father and mother, who had suffered great anxiety for them
Establishing a good predictive model for risk stratification of many genetic variants predisposing to CRC is important for more targeted screening of high-risk patients.A study using a polygenic risk score (PRS) derived from 95 common genetic variants was able to predict the risk of EOCRC when testing 12197 early-onset CRC and 95865 lateonset CRC patients of European descent.A higher PRS is more strongly associated with EOCRC than late-onset patients.Those in the highest PRS quartile had a 3.7-fold increased risk of EOCRC compared with those in the lowest quartile.Interestingly,high PRS cases also had a tendency towards distal and rectal tumors[78].PRS may therefore be a useful tool to stratify risk when used alongside the identification of other lifestyle and environmental risk factors,and may pick up some high risk patients within the average-risk screening group who would otherwise have not been identified based on conventional criteria.This may provide a more targeted and personalized approach for CRC screening than our current standard of care.
She wove one month, she wove two months-all the winter Vasilissa sat weaving, weaving her fine thread, till the whole piece of linen46 was done, of a texture47 so fine that it could be passed, like thread, through the eye of a needle. When the spring came she bleached48 it, so white that no snow could be compared with it. Then she said to the old woman: Take thou the linen to the market, grandmothers and sell it, and the money shall suffice to pay for my food and lodging49. When the old woman examined the linen, however, she said:
CONCLUSION
CRC is a genetic and molecularly heterogeneous disease.EOCRC represents a subgroup of CRC with unique characteristics.Genetic predisposition and multiple risk factors are being explored as potential contributors to this rising trend.Given the long process of transition from non-neoplastic cells to malignancy,exploring early-life exposures as potential culprits is important[13].Increasing evidence has shown that obesity,sedentary lifestyle,Westernized diet,and high sugar intake are significant risk factors for EOCRC.Exposures as early as in the prenatal or perinatal stages of life,such as maternal diet or delivery methods,have been postulated to affect the composition of the gut microbiota.However,studies that prove causality remain elusive.Large epidemiological studies are still needed to further discover or verify potential causative factors.
The relationship between diet,lifestyle,and gut dysbiosis and their respective roles in colorectal carcinogenesis are complex.The composition of gut microbiome is dynamic and dependent on multiple factors including race,age,lifestyle,diet,medication use,stress,
There is currently no clear consensus for the definition of gut dysbiosis due to the high microbial heterogeneity in CRC[88].Further investigations on the gut microenvironment from stool samples in CRC patients may help characterize the gut microbiome that predisposes to CRC,with emerging evidence that shows promise for its use in CRC screening and risk stratification.Future research on manipulating the gut microbiome through diet or drugs like probiotics may even play a role in cancer prevention.
This “l(fā)iterature” has to be read and signed, or placed at the bottom of the birdcage. Regardless of its destination it must be dealt with on a daily basis.
Apart from the need for further research on exploring the unanswered questions of the underlying cause and mechanisms behind EOCRC,numerous barriers to the reduction of the incidence of EOCRC still exist.Poor compliance with early screening programs may be due to inadequate public awareness[89].Information on family history may not be known to patients.Young patients and physicians alike tend to attribute early symptoms to non-sinister pathologies that may result in a delay of diagnosis.A study of young patients has shown that they present to a medical practitioner on average 294 d after the onset of rectal bleeding,which likely resulted in a more advanced stage of disease[90].With regard to healthcare systems,there may be access,cost,or policy barriers to screening and treatment.
Steps to fight EOCRC include raising awareness of this growing threat through education and public promotion.This includes public awareness campaigns,educating the public on the dietary or lifestyle risks of CRC,and enhancing physician awareness of EOCRC.Advising young patients to stay vigilant of early symptoms,such as per-rectal bleeding,abdominal pain,weight loss,and change in bowel habits and to seek timely medical attention is also important.Promoting awareness of early colonoscopy screening for high-risk groups,and referring patients who are eligible for genetic counselling and testing are essential for early identification of at-risk individuals.Further research on predisposing genetic and epigenetic signatures is needed.In the future,we should strive for specific genetic profiling through wholegenome sequencing for better risk stratification[91].It may be useful to see how well specific risk stratification tools including lifestyle risks or PRS perform in the real world to identify high-risk patients for a more personalized screening strategy,which in turn may allow for better allocation of resources to those most in need to combat this global rise in EOCRC.
1 Cancer Today.Global Cancer Observatory.International Agency for Research on Cancer.Available from:https://gco.iarc.fr/today/fact-sheets-cancers
2 Surveillance,Epidemiology,and End Results.Cancer of the Colon and Rectum-Cancer Stat Facts.Available from:https://seer.cancer.gov/statfacts/html/colorect.html
3 Siegel RL,Torre LA,Soerjomataram I,Hayes RB,Bray F,Weber TK,Jemal A.Global patterns and trends in colorectal cancer incidence in young adults.
2019;68:2179-2185 [PMID:31488504 DOI:10.1136/gutjnl-2019-319511]
4 Lui RN,Tsoi KKF,Ho JMW,Lo CM,Chan FCH,Kyaw MH,Sung JJY.Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents:A Joinpoint Regression Analysis of 1,922,167 Cases.
2019;28:1275-1282 [PMID:31113868 DOI:10.1158/1055-9965.EPI-18-1111]
5 Sung JJY,Chiu HM,Jung KW,Jun JK,Sekiguchi M,Matsuda T,Kyaw MH.Increasing Trend in Young-Onset Colorectal Cancer in Asia:More Cancers in Men and More Rectal Cancers.
2019;114:322-329 [PMID:30694865 DOI:10.14309/ajg.0000000000000133]
6 Saad El Din K,Loree JM,Sayre EC,Gill S,Brown CJ,Dau H,De Vera MA.Trends in the epidemiology of young-onset colorectal cancer:a worldwide systematic review.
2020;20:288 [PMID:32252672 DOI:10.1186/s12885-020-06766-9]
7 Abualkhair WH,Zhou M,Ahnen D,Yu Q,Wu XC,Karlitz JJ.Trends in Incidence of Early-Onset Colorectal Cancer in the United States Among Those Approaching Screening Age.
2020;3:e1920407 [PMID:32003823 DOI:10.1001/jamanetworkopen.2019.20407]
8 Peterse EFP,Meester RGS,Siegel RL,Chen JC,Dwyer A,Ahnen DJ,Smith RA,Zauber AG,Lansdorp-Vogelaar I.The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening:Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline.
2018;124:2964-2973 [PMID:29846933 DOI:10.1002/cncr.31543]
9 O'Connell JB,Maggard MA,Liu JH,Etzioni DA,Livingston EH,Ko CY.Rates of colon and rectal cancers are increasing in young adults.
2003;69:866-872 [PMID:14570365 DOI:10.1046/j.1600-6143.2003.00209.x]
10 Mauri G,Sartore-Bianchi A,Russo AG,Marsoni S,Bardelli A,Siena S.Early-onset colorectal cancer in young individuals.
2019;13:109-131 [PMID:30520562 DOI:10.1002/1878-0261.12417]
11 Khan M,Korphaisarn K,Saif A,Foo WC,Kopetz S.Early-Onset Signet-Ring Cell Adenocarcinoma of the Colon:A Case Report and Review of the Literature.
2017;2017:2832180[PMID:28326211 DOI:10.1155/2017/2832180]
12 Wells K,Wise PE.Hereditary Colorectal Cancer Syndromes.
2017;97:605-625[PMID:28501250 DOI:10.1016/j.suc.2017.01.009]
13 Hofseth LJ,Hebert JR,Chanda A,Chen H,Love BL,Pena MM,Murphy EA,Sajish M,Sheth A,Buckhaults PJ,Berger FG.Early-onset colorectal cancer:initial clues and current views.
2020;17:352-364 [PMID:32086499 DOI:10.1038/s41575-019-0253-4]
14 Rawla P,Sunkara T,Barsouk A.Epidemiology of colorectal cancer:incidence,mortality,survival,and risk factors.
2019;14:89-103 [PMID:31616522 DOI:10.5114/pg.2018.81072]
15 Jiang Y,Ben Q,Shen H,Lu W,Zhang Y,Zhu J.Diabetes mellitus and incidence and mortality of colorectal cancer:a systematic review and meta-analysis of cohort studies.
2011;26:863-876 [PMID:21938478 DOI:10.1007/s10654-011-9617-y]
16 O'Sullivan DE,Sutherland RL,Town S,Chow K,Fan J,Forbes N,Heitman SJ,Hilsden RJ,Brenner DR.Risk Factors for Early-Onset Colorectal Cancer:A Systematic Review and Meta-analysis.
2021 [PMID:33524598 DOI:10.1016/j.cgh.2021.01.037]
17 Carethers JM.Screening for colorectal cancer in African Americans:determinants and rationale for an earlier age to commence screening.
2015;60:711-721 [PMID:25540085 DOI:10.1007/s10620-014-3443-5]
18 Shaukat A,Kahi CJ,Burke CA,Rabeneck L,Sauer BG,Rex DK.ACG Clinical Guidelines:Colorectal Cancer Screening 2021.
2021;116:458-479 [PMID:33657038 DOI:10.14309/ajg.0000000000001122]
19 Muller C,Ihionkhan E,Stoffel EM,Kupfer SS.Disparities in Early-Onset Colorectal Cancer.
2021;10 [PMID:33925893 DOI:10.3390/cells10051018]
20 Murphy CC,Wallace K,Sandler RS,Baron JA.Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival.
2019;156:958-965 [PMID:30521807 DOI:10.1053/j.gastro.2018.11.060]
21 Ma Y,Yang Y,Wang F,Zhang P,Shi C,Zou Y,Qin H.Obesity and risk of colorectal cancer:a systematic review of prospective studies.
2013;8:e53916 [PMID:23349764 DOI:10.1371/journal.pone.0053916]
22 Hussan H,Patel A,Hinton A,Ma Q,Tabung,Fred KT,Clinton S.The Associations Between Obesity and Early Onset Colorectal Cancer:A Propensity-Weighted Analysis of the National Health and Nutrition Examination Survey (NHANES).
2020;115 [DOI:10.14309/01.ajg.0000706892.59075.79]
23 Kyrgiou M,Kalliala I,Markozannes G,Gunter MJ,Paraskevaidis E,Gabra H,Martin-Hirsch P,Tsilidis KK.Adiposity and cancer at major anatomical sites:umbrella review of the literature.
2017;356:j477 [PMID:28246088 DOI:10.1136/bmj.j477]
24 Kim JY,Jung YS,Park JH,Kim HJ,Cho YK,Sohn CI,Jeon WK,Kim BI,Choi KY,Park DI.Different risk factors for advanced colorectal neoplasm in young adults.
2016;22:3611-3620 [PMID:27053853 DOI:10.3748/wjg.v22.i13.3611]
25 Liu PH,Wu K,Ng K,Zauber AG,Nguyen LH,Song M,He X,Fuchs CS,Ogino S,Willett WC,Chan AT,Giovannucci EL,Cao Y.Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women.
2019;5:37-44 [PMID:30326010 DOI:10.1001/jamaoncol.2018.4280]
26 Ye P,Xi Y,Huang Z,Xu P.Linking Obesity with Colorectal Cancer:Epidemiology and Mechanistic Insights.
2020;12 [PMID:32486076 DOI:10.3390/cancers12061408]
27 Park JH,Moon JH,Kim HJ,Kong MH,Oh YH.Sedentary Lifestyle:Overview of Updated Evidence of Potential Health Risks.
2020;41:365-373 [PMID:33242381 DOI:10.4082/kjfm.20.0165]
28 Nguyen LH,Liu PH,Zheng X,Keum N,Zong X,Li X,Wu K,Fuchs CS,Ogino S,Ng K,Willett WC,Chan AT,Giovannucci EL,Cao Y.Sedentary Behaviors,TV Viewing Time,and Risk of Young-Onset Colorectal Cancer.
2018;2:pky073 [PMID:30740587 DOI:10.1093/jncics/pky073]
29 Brennan CA,Garrett WS.Gut Microbiota,Inflammation,and Colorectal Cancer.
2016;70:395-411 [PMID:27607555 DOI:10.1146/annurev-micro-102215-095513]
30 Bressa C,Bailén-Andrino M,Pérez-Santiago J,González-Soltero R,Pérez M,Montalvo-Lominchar MG,Maté-Mu?oz JL,Domínguez R,Moreno D,Larrosa M.Differences in gut microbiota profile between women with active lifestyle and sedentary women.
2017;12:e0171352 [PMID:28187199 DOI:10.1371/journal.pone.0171352]
31 Mach N,Fuster-Botella D.Endurance exercise and gut microbiota:A review.
2017;6:179-197 [PMID:30356594 DOI:10.1016/j.jshs.2016.05.001]
32 Castelló A,Amiano P,Fernández de Larrea N,Martín V,Alonso MH,Casta?o-Vinyals G,Pérez-Gómez B,Olmedo-Requena R,Guevara M,Fernandez-Tardon G,Dierssen-Sotos T,Llorens-Ivorra C,Huerta JM,Capelo R,Fernández-Villa T,Díez-Villanueva A,Urtiaga C,Castilla J,Jiménez-Moleón JJ,Moreno V,Dávila-Batista V,Kogevinas M,Aragonés N,Pollán M;MCC-Spain researchers.Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer.
2019;58:1495-1505 [PMID:29582162 DOI:10.1007/s00394-018-1674-5]
33 Feng YL,Shu L,Zheng PF,Zhang XY,Si CJ,Yu XL,Gao W,Zhang L.Dietary patterns and colorectal cancer risk:a meta-analysis.
2017;26:201-211 [PMID:26945285 DOI:10.1097/CEJ.0000000000000245]
34 Albracht-Schulte K,Islam T,Johnson P,Moustaid-Moussa N.Systematic Review of Beef Protein Effects on Gut Microbiota:Implications for Health.
2021;12:102-114 [PMID:32761179 DOI:10.1093/advances/nmaa085]
35 Larsson SC,Wolk A.Meat consumption and risk of colorectal cancer:a meta-analysis of prospective studies.
2006;119:2657-2664 [PMID:16991129 DOI:10.1002/ijc.22170]
36 Chan DS,Lau R,Aune D,Vieira R,Greenwood DC,Kampman E,Norat T.Red and processed meat and colorectal cancer incidence:meta-analysis of prospective studies.
2011;6:e20456[PMID:21674008 DOI:10.1371/journal.pone.0020456]
37 Zhang Q,Wang Y,Fu L.Dietary advanced glycation end-products:Perspectives linking food processing with health implications.
2020;19:2559-2587 [PMID:33336972 DOI:10.1111/1541-4337.12593]
38 Omofuma OO,Turner DP,Peterson LL,Merchant AT,Zhang J,Steck SE.Dietary Advanced Glycation End-products (AGE) and Risk of Breast Cancer in the Prostate,Lung,Colorectal and Ovarian Cancer Screening Trial (PLCO).
2020;13:601-610 [PMID:32169887 DOI:10.1158/1940-6207.CAPR-19-0457]
39 Azizian-Farsani F,Abedpoor N,Hasan Sheikhha M,Gure AO,Nasr-Esfahani MH,Ghaedi K.Receptor for Advanced Glycation End Products Acts as a Fuel to Colorectal Cancer Development.
2020;10:552283 [PMID:33117687 DOI:10.3389/fonc.2020.552283]
40 Lopez-Moreno J,Quintana-Navarro GM,Delgado-Lista J,Garcia-Rios A,Delgado-Casado N,Camargo A,Perez-Martinez P,Striker GE,Tinahones FJ,Perez-Jimenez F,Lopez-Miranda J,Yubero-Serrano EM.Mediterranean Diet Reduces Serum Advanced Glycation End Products and Increases Antioxidant Defenses in Elderly Adults:A Randomized Controlled Trial.
2016;64:901-904 [PMID:27100598 DOI:10.1111/jgs.14062]
41 Farinetti A,Zurlo V,Manenti A,Coppi F,Mattioli AV.Mediterranean diet and colorectal cancer:A systematic review.
2017;43-44:83-88 [PMID:28935150 DOI:10.1016/j.nut.2017.06.008]
42 Grosso G,Biondi A,Galvano F,Mistretta A,Marventano S,Buscemi S,Drago F,Basile F.Factors associated with colorectal cancer in the context of the Mediterranean diet:a case-control study.
2014;66:558-565 [PMID:24754383 DOI:10.1080/01635581.2014.902975]
43 Zheng X,Hur J,Nguyen LH,Liu J,Song M,Wu K,Smith-Warner SA,Ogino S,Willett WC,Chan AT,Giovannucci E,Cao Y.Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer.
2021;113:543-552 [PMID:33136160 DOI:10.1093/jnci/djaa164]
44 Mehta RS,Song M,Nishihara R,Drew DA,Wu K,Qian ZR,Fung TT,Hamada T,Masugi Y,da Silva A,Shi Y,Li W,Gu M,Willett WC,Fuchs CS,Giovannucci EL,Ogino S,Chan AT.Dietary Patterns and Risk of Colorectal Cancer:Analysis by Tumor Location and Molecular Subtypes.
2017;152:1944-1953.e1 [PMID:28249812 DOI:10.1053/j.gastro.2017.02.015]
45 Veettil SK,Wong TY,Loo YS,Playdon MC,Lai NM,Giovannucci EL,Chaiyakunapruk N.Role of Diet in Colorectal Cancer Incidence:Umbrella Review of Meta-analyses of Prospective Observational Studies.
2021;4:e2037341 [PMID:33591366 DOI:10.1001/jamanetworkopen.2020.37341]
46 Zheng X,Wu K,Song M,Ogino S,Fuchs CS,Chan AT,Giovannucci EL,Cao Y,Zhang X.Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer.
2020;69:970-972 [PMID:31209182 DOI:10.1136/gutjnl-2019-318374]
47 Shahbandeh M.Global Sugar Consumption,2020/21.Statista.2021 May 27.Available from:https://www.statista.com/statistics/249681/total-consumption-of-sugar-worldwide/
48 OECD iLibrary.OECD-FAO Agricultural Outlook 2019-2028.OECD Publishing.2019.Available from:https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2019-2028_bdef14fa-en
49 Hur J,Otegbeye E,Joh HK,Nimptsch K,Ng K,Ogino S,Meyerhardt JA,Chan AT,Willett WC,Wu K,Giovannucci E,Cao Y.Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women.
2021 [PMID:33958435 DOI:10.1136/gutjnl-2020-323450]
50 Slattery ML,Benson J,Berry TD,Duncan D,Edwards SL,Caan BJ,Potter JD.Dietary sugar and colon cancer.
1997;6:677-685 [PMID:9298574 DOI:10.1016/S0278-6915(97)85474-9]
51 Malik VS,Popkin BM,Bray GA,Després JP,Hu FB.Sugar-sweetened beverages,obesity,type 2 diabetes mellitus,and cardiovascular disease risk.
2010;121:1356-1364 [PMID:20308626 DOI:10.1161/CIRCULATIONAHA.109.876185]
52 Zhao S,Jang C,Liu J,Uehara K,Gilbert M,Izzo L,Zeng X,Trefely S,Fernandez S,Carrer A,Miller KD,Schug ZT,Snyder NW,Gade TP,Titchenell PM,Rabinowitz JD,Wellen KE.Dietary fructose feeds hepatic lipogenesis
microbiota-derived acetate.
2020;579:586-591 [PMID:32214246 DOI:10.1038/s41586-020-2101-7]
53 Sotokawauchi A,Matsui T,Higashimoto Y,Yamagishi SI.Fructose causes endothelial cell damage
activation of advanced glycation end products-receptor system.
2019;16:556-561 [PMID:31375034 DOI:10.1177/1479164119866390]
54 Crowe W,Elliott CT,Green BD.A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer.
2019;11 [PMID:31694233 DOI:10.3390/nu11112673]
55 Dahmus JD,Kotler DL,Kastenberg DM,Kistler CA.The gut microbiome and colorectal cancer:a review of bacterial pathogenesis.
2018;9:769-777 [PMID:30151274 DOI:10.21037/jgo.2018.04.07]
56 B?ckhed F,Roswall J,Peng Y,Feng Q,Jia H,Kovatcheva-Datchary P,Li Y,Xia Y,Xie H,Zhong H,Khan MT,Zhang J,Li J,Xiao L,Al-Aama J,Zhang D,Lee YS,Kotowska D,Colding C,Tremaroli V,Yin Y,Bergman S,Xu X,Madsen L,Kristiansen K,Dahlgren J,Wang J.Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life.
2015;17:690-703 [PMID:25974306 DOI:10.1016/j.chom.2015.04.004]
57 Thursby E,Juge N.Introduction to the human gut microbiota.
2017;474:1823-1836[PMID:28512250 DOI:10.1042/BCJ20160510]
58 Moore RE,Townsend SD.Temporal development of the infant gut microbiome.
2019;9:190128 [PMID:31506017 DOI:10.1098/rsob.190128]
59 O'Keefe SJ,Ou J,Aufreiter S,O'Connor D,Sharma S,Sepulveda J,Fukuwatari T,Shibata K,Mawhinney T.Products of the colonic microbiota mediate the effects of diet on colon cancer risk.
2009;139:2044-2048 [PMID:19741203 DOI:10.3945/jn.109.104380]
60 Chen J,Vitetta L.The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation.
2020;20:e15 [PMID:32395367 DOI:10.4110/in.2020.20.e15]
61 Tilg H,Adolph TE,Gerner RR,Moschen AR.The Intestinal Microbiota in Colorectal Cancer.
2018;33:954-964 [PMID:29657127 DOI:10.1016/j.ccell.2018.03.004]
62 Zhu G,Huang Q,Huang Y,Zheng W,Hua J,Yang S,Zhuang J,Wang J,Ye J.Lipopolysaccharide increases the release of VEGF-C that enhances cell motility and promotes lymphangiogenesis and lymphatic metastasis through the TLR4-NF-κB/JNK pathways in colorectal cancer.
2016;7:73711-73724 [PMID:27713159 DOI:10.18632/oncotarget.12449]
63 Oliver A,Chase AB,Weihe C,Orchanian SB,Riedel SF,Hendrickson CL,Lay M,Sewall JM,Martiny JBH,Whiteson K.High-Fiber,Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids.
2021;6 [PMID:33727392 DOI:10.1128/mSystems.00115-21]
64 Bahmani S,Azarpira N,Moazamian E.Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742.
2019;30:835-842 [PMID:31530527 DOI:10.5152/tjg.2019.18451]
65 Wei H,Chen L,Lian G,Yang J,Li F,Zou Y,Lu F,Yin Y.Antitumor mechanisms of bifidobacteria.
2018;16:3-8 [PMID:29963126 DOI:10.3892/ol.2018.8692]
66 Swanson KS,Gibson GR,Hutkins R,Reimer RA,Reid G,Verbeke K,Scott KP,Holscher HD,Azad MB,Delzenne NM,Sanders ME.The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics.
2020;17:687-701 [PMID:32826966 DOI:10.1038/s41575-020-0344-2]
67 Dikeocha IJ,Al-Kabsi AM,Hussin S,Alshawsh MA.Role of probiotics in patients with colorectal cancer:a systematic review protocol of randomised controlled trial studies.
2020;10:e038128 [PMID:32771989 DOI:10.1136/bmjopen-2020-038128]
68 Dos Reis SA,da Concei??o LL,Siqueira NP,Rosa DD,da Silva LL,Peluzio MD.Review of the mechanisms of probiotic actions in the prevention of colorectal cancer.
2017;37:1-19[PMID:28215310 DOI:10.1016/j.nutres.2016.11.009]
69 Cruz BCS,Sarandy MM,Messias AC,Gon?alves RV,Ferreira CLLF,Peluzio MCG.Preclinical and clinical relevance of probiotics and synbiotics in colorectal carcinogenesis:a systematic review.
2020;78:667-687 [PMID:31917829 DOI:10.1093/nutrit/nuz087]
70 Sivamaruthi BS,Kesika P,Chaiyasut C.The Role of Probiotics in Colorectal Cancer Management.
2020;2020:3535982 [PMID:32148539 DOI:10.1155/2020/3535982]
71 Daca Alvarez M,Quintana I,Terradas M,Mur P,Balaguer F,Valle L.The Inherited and Familial Component of Early-Onset Colorectal Cancer.
2021;10 [PMID:33806975 DOI:10.3390/cells10030710]
72 Wilkins T,McMechan D,Talukder A,Herline A.Colorectal Cancer Screening and Surveillance in Individuals at Increased Risk.
2018;97:111-116 [PMID:29365221]
73 Perrod G,Rahmi G,Cellier C.Colorectal cancer screening in Lynch syndrome:Indication,techniques and future perspectives.
2021;33:520-528 [PMID:32314431 DOI:10.1111/den.13702]
74 van Leerdam ME,Roos VH,van Hooft JE,Dekker E,Jover R,Kaminski MF,Latchford A,Neumann H,Pellisé M,Saurin JC,Tanis PJ,Wagner A,Balaguer F,Ricciardiello L.Endoscopic management of polyposis syndromes:European Society of Gastrointestinal Endoscopy (ESGE)Guideline.
2019;51:877-895 [PMID:31342472 DOI:10.1055/a-0965-0605]
75 Gupta S,Bharti B,Ahnen DJ,Buchanan DD,Cheng IC,Cotterchio M,Figueiredo JC,Gallinger SJ,Haile RW,Jenkins MA,Lindor NM,Macrae FA,Le Marchand L,Newcomb PA,Thibodeau SN,Win AK,Martinez ME.Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer.
2020;126:3013-3020 [PMID:32307706 DOI:10.1002/cncr.32851]
76 Pearlman R,Frankel WL,Swanson B,Zhao W,Yilmaz A,Miller K,Bacher J,Bigley C,Nelsen L,Goodfellow PJ,Goldberg RM,Paskett E,Shields PG,Freudenheim JL,Stanich PP,Lattimer I,Arnold M,Liyanarachchi S,Kalady M,Heald B,Greenwood C,Paquette I,Prues M,Draper DJ,Lindeman C,Kuebler JP,Reynolds K,Brell JM,Shaper AA,Mahesh S,Buie N,Weeman K,Shine K,Haut M,Edwards J,Bastola S,Wickham K,Khanduja KS,Zacks R,Pritchard CC,Shirts BH,Jacobson A,Allen B,de la Chapelle A,Hampel H;Ohio Colorectal Cancer Prevention Initiative Study Group.Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer.
2017;3:464-471 [PMID:27978560 DOI:10.1001/jamaoncol.2016.5194]
77 Thomas M,Sakoda LC,Hoffmeister M,Rosenthal EA,Lee JK,van Duijnhoven FJB,Platz EA,Wu AH,Dampier CH,de la Chapelle A,Wolk A,Joshi AD,Burnett-Hartman A,Gsur A,Lindblom A,Castells A,Win AK,Namjou B,Van Guelpen B,Tangen CM,He Q,Li CI,Schafmayer C,Joshu CE,Ulrich CM,Bishop DT,Buchanan DD,Schaid D,Drew DA,Muller DC,Duggan D,Crosslin DR,Albanes D,Giovannucci EL,Larson E,Qu F,Mentch F,Giles GG,Hakonarson H,Hampel H,Stanaway IB,Figueiredo JC,Huyghe JR,Minnier J,Chang-Claude J,Hampe J,Harley JB,Visvanathan K,Curtis KR,Offit K,Li L,Le Marchand L,Vodickova L,Gunter MJ,Jenkins MA,Slattery ML,Lemire M,Woods MO,Song M,Murphy N,Lindor NM,Dikilitas O,Pharoah PDP,Campbell PT,Newcomb PA,Milne RL,MacInnis RJ,Castellví-Bel S,Ogino S,Berndt SI,Bézieau S,Thibodeau SN,Gallinger SJ,Zaidi SH,Harrison TA,Keku TO,Hudson TJ,Vymetalkova V,Moreno V,Martín V,Arndt V,Wei WQ,Chung W,Su YR,Hayes RB,White E,Vodicka P,Casey G,Gruber SB,Schoen RE,Chan AT,Potter JD,Brenner H,Jarvik GP,Corley DA,Peters U,Hsu L.Genomewide Modeling of Polygenic Risk Score in Colorectal Cancer Risk.
2020;107:432-444 [PMID:32758450 DOI:10.1016/j.ajhg.2020.07.006]
78 Archambault AN,Su YR,Jeon J,Thomas M,Lin Y,Conti DV,Win AK,Sakoda LC,Lansdorp-Vogelaar I,Peterse EFP,Zauber AG,Duggan D,Holowatyj AN,Huyghe JR,Brenner H,Cotterchio M,Bézieau S,Schmit SL,Edlund CK,Southey MC,MacInnis RJ,Campbell PT,Chang-Claude J,Slattery ML,Chan AT,Joshi AD,Song M,Cao Y,Woods MO,White E,Weinstein SJ,Ulrich CM,Hoffmeister M,Bien SA,Harrison TA,Hampe J,Li CI,Schafmayer C,Offit K,Pharoah PD,Moreno V,Lindblom A,Wolk A,Wu AH,Li L,Gunter MJ,Gsur A,Keku TO,Pearlman R,Bishop DT,Castellví-Bel S,Moreira L,Vodicka P,Kampman E,Giles GG,Albanes D,Baron JA,Berndt SI,Brezina S,Buch S,Buchanan DD,Trichopoulou A,Severi G,Chirlaque MD,Sánchez MJ,Palli D,Kühn T,Murphy N,Cross AJ,Burnett-Hartman AN,Chanock SJ,de la Chapelle A,Easton DF,Elliott F,English DR,Feskens EJM,FitzGerald LM,Goodman PJ,Hopper JL,Hudson TJ,Hunter DJ,Jacobs EJ,Joshu CE,Küry S,Markowitz SD,Milne RL,Platz EA,Rennert G,Rennert HS,Schumacher FR,Sandler RS,Seminara D,Tangen CM,Thibodeau SN,Toland AE,van Duijnhoven FJB,Visvanathan K,Vodickova L,Potter JD,M?nnist? S,Weigl K,Figueiredo J,Martín V,Larsson SC,Parfrey PS,Huang WY,Lenz HJ,Castelao JE,Gago-Dominguez M,Mu?oz-Garzón V,Mancao C,Haiman CA,Wilkens LR,Siegel E,Barry E,Younghusband B,Van Guelpen B,Harlid S,Zeleniuch-Jacquotte A,Liang PS,Du M,Casey G,Lindor NM,Le Marchand L,Gallinger SJ,Jenkins MA,Newcomb PA,Gruber SB,Schoen RE,Hampel H,Corley DA,Hsu L,Peters U,Hayes RB.Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer.
2020;158:1274-1286.e12 [PMID:31866242 DOI:10.1053/j.gastro.2019.12.012]
79 Mork ME,You YN,Ying J,Bannon SA,Lynch PM,Rodriguez-Bigas MA,Vilar E.High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer.
2015;33:3544-3549 [PMID:26195711 DOI:10.1200/JCO.2015.61.4503]
80 Nguyen HT,Duong HQ.The molecular characteristics of colorectal cancer:Implications for diagnosis and therapy.
2018;16:9-18 [PMID:29928381 DOI:10.3892/ol.2018.8679]
81 Yurgelun MB,Kulke MH,Fuchs CS,Allen BA,Uno H,Hornick JL,Ukaegbu CI,Brais LK,McNamara PG,Mayer RJ,Schrag D,Meyerhardt JA,Ng K,Kidd J,Singh N,Hartman AR,Wenstrup RJ,Syngal S.Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer.
2017;35:1086-1095 [PMID:28135145 DOI:10.1200/JCO.2016.71.0012]
82 Jang E,Chung DC.Hereditary colon cancer:lynch syndrome.
2010;4:151-160 [PMID:20559516 DOI:10.5009/gnl.2010.4.2.151]
83 Tapial S,Olmedillas-López S,Rueda D,Arriba M,García JL,Vivas A,Pérez J,Pena-Couso L,Olivera R,Rodríguez Y,García-Arranz M,García-Olmo D,González-Sarmiento R,Urioste M,Goel A,Perea J.Cimp-Positive Status is More Representative in Multiple Colorectal Cancers than in Unique Primary Colorectal Cancers.
2019;9:10516 [PMID:31324877 DOI:10.1038/s41598-019-47014-w]
84 Willauer AN,Liu Y,Pereira AAL,Lam M,Morris JS,Raghav KPS,Morris VK,Menter D,Broaddus R,Meric-Bernstam F,Hayes-Jordan A,Huh W,Overman MJ,Kopetz S,Loree JM.Clinical and molecular characterization of early-onset colorectal cancer.
2019;125:2002-2010[PMID:30854646 DOI:10.1002/cncr.31994]
85 Magnani G,Furlan D,Sahnane N,Reggiani Bonetti L,Domati F,Pedroni M.Molecular Features and Methylation Status in Early Onset (≤40 Years) Colorectal Cancer:A Population Based,Case-Control Study.
2015;2015:132190 [PMID:26557847 DOI:10.1155/2015/132190]
86 Ribic CM,Sargent DJ,Moore MJ,Thibodeau SN,French AJ,Goldberg RM,Hamilton SR,Laurent-Puig P,Gryfe R,Shepherd LE,Tu D,Redston M,Gallinger S.Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer.
2003;349:247-257 [PMID:12867608 DOI:10.1056/NEJMoa022289]
87 Slomski A.Aspirin Protects Against Colorectal Cancer in Lynch Syndrome.
2020;324:733[PMID:32840595 DOI:10.1001/jama.2020.14804]
88 Kosumi K,Mima K,Baba H,Ogino S.Dysbiosis of the gut microbiota and colorectal cancer:the key target of molecular pathological epidemiology.
2018;3 [PMID:30345420 DOI:10.21037/jlpm.2018.09.05]
89 Hogan NM,Hanley M,Hogan AM,Sheehan M,McAnena OJ,Regan MP,Kerin MJ,Joyce MR.Awareness and uptake of family screening in patients diagnosed with colorectal cancer at a young age.
2015;2015:194931 [PMID:25688262 DOI:10.1155/2015/194931]
90 Sandhu GS,Anders R,Walde A,Leal AD,King GT,Leong S,Davis SL,Purcell WT,Goodman KA,Herter W,Meguid CL,Birnbaum EH,Ahrendt SA,Gleisner A,Schulick RD,Delchiaro M,McCarter M,Patel S,Messersmith WA,Lieu CH.High incidence of advanced stage cancer and prolonged rectal bleeding history before diagnosis in young-onset patients with colorectal cancer.
2019 37:3576-3576 [DOI:10.1200/JCO.2019.37.15_suppl.3576]
91 Valle L,de Voer RM,Goldberg Y,Sjursen W,F?rsti A,Ruiz-Ponte C,Caldés T,Garré P,Olsen MF,Nordling M,Castellvi-Bel S,Hemminki K.Update on genetic predisposition to colorectal cancer and polyposis.
2019;69:10-26 [PMID:30862463 DOI:10.1016/j.mam.2019.03.001]
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
