Trimodality treatment in gastric and gastroesophageal junction cancers:Current approach and future perspectives
INTRODUCTION
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer mortality with a varying incidence worldwide[1].The highest incidence (>20 per 100000 in men) is seen in China,Japan,Korea,Latin America and Eastern Europe,whereas the lowest incidence (<10 per 100000 in men) is seen in North America,parts of Africa and Northern Europe[2].In the West,cancers located at the gastroesophageal junction (GEJ) are less frequent than in the stomach,however,incidence of GEJ cancer has been steadily rising during the last decade[3].Only 27% of newly diagnosed GCs are localized with a 5-year overall survival (OS) rate of 30.4%,which,unfortunately,remains stable over the last 30 to 40 years[4].Surgery is still the only chance for cure and implementation of a multimodality treatment approach is utilized to further improve survival.Advanced disease carries a dismal prognosis and treatment remains challenging with a 5-year OS rate less than 5%.Thus,despite decreasing incidence,GC and GEJ cancers remain a serious health burden globally with high mortality rates.
Histology
The vast majority (95%) of gastric malignant neoplasms are adenocarcinomas,which are typically classified based on anatomic location and histologic subtype by Lauren as intestinal and diffuse[5].Two different mechanisms of carcinogenesis have been implicated for GC correlating with the two different histologic subtypes.Intestinaltype gastric adenocarcinoma has been heavily associated with Helicobacter pylori infection as well as other environmental factors,such as alcohol,processed meat,smoking,and obesity.Diffuse-type gastric adenocarcinoma usually arises from defective intracellular adhesion molecules due to loss of E-cadherin protein expression that is encoded bygene[6].Genomic mutation ingene is the cause of the Hereditary Diffuse Gastric Cancer Syndrome,which increases the chance for diffuse gastric cancer throughout a person’s lifetime up to 70%[7].
Esophageal cancers are histologically diverse and can be either squamous cell carcinomas (SCC) or adenocarcinomas.Carcinomas of the GEJ are classified into the esophageal cancer group according to the 8edition of American Joint Committee on Cancer/Union for International Cancer Control[8].According to their histology and molecular characteristics,GEJ tumors may be further grouped within either esophageal or gastric cancer groups.Specifically,adenocarcinomas of the GEJ share similar molecular traits with distal esophageal adenocarcinoma and upper gastric adenocarcinoma,as highlighted in the Cancer Genome Atlas analysis discussed further in the article[9].Each histologic subtype differs in terms of primary tumor location and somewhat in prognosis[10].Esophageal SCC arises from the squamous lining of the esophagus through progression of premalignant precursor lesions that occur in the presence of risk factors that cause chronic irritation and inflammation,is more likely to localize near the tracheal bifurcation,has a proclivity for earlier lymphatic spread,and is associated with a poorer prognosis[11].Tobacco and alcohol consumption are major risk factors for SCC,whereas tobacco use is a moderate risk factor for adenocarcinoma.SCC usually arises in the middle third of the esophagus (or higher),followed by the lower third and then less commonly to the upper third[12,13].Adenocarcinomas arise in the distal esophagus or the GEJ and have been positively associated with GERD,obesity with an increased risk in people with BMI over 30 kg/m[14].This can be attributed partially to the increased risk of gastroesophageal reflux disease in obese individuals,which may lead to development of Barrett esophagus,a pre-malignant condition in which regular squamous epithelium of the esophagus is replaced by metaplastic columnar epithelium[15].
Molecular classifications
Apart from the traditional histologic subtypes,analysis from TCGA project has also classified GC into 4 different categories based on their genomic profile[4]:Tumors containing Epstein–Barr virus (EBV) account for approximately 10% of GCs and are characterized by high prevalence of DNA hypermethylation,amplification of,andgenes.Moreover,nearly 80% have an amino acid-changing alteration in thegene.EBV-associated tumors are usually located in the proximal stomach and are associated with non-diffuse type.Large meta-analyses of multiple multicenter studies have concluded that EBV (+) GC has more favorable outcomes in comparison to EBV (-)[16].Furthermore,Sohn[17] concluded that EBV (+) GC has the best prognosis among all other subtypes.
Microsatellite instability (MSI-H) is present in around 20% of GCs.Tumors showing mismatch repair deficiency contain a high rate of mutations,including mutations of genes encoding targetable oncogenic proteins and take place due to malfunctioning in the DNA repair mechanisms.These tumors are characterized by MLH1 hypermethylation and CIMP[18].In terms of prognosis,MSI GC has worse prognosis than EBV (+)and better than genomically stable (GS) subtype,according to Sohn’s prognostic model[17].Finally,patients with MSI-H and EBV positive tumors have shown high rates of response to immunotherapy and durable survival outcomes[18].
A phase II study by Ku[72] examined the effectiveness of adding the anti-VEGF antibody bevacizumab to preoperative induction chemotherapy and chemoradiation with cisplatin and irinotecan in patients with resectable locally advanced esophageal and GEJ adenocarcinomas.The final evaluable population of the study consisted of 33 patients,all with cancer of the GEJ.25 patients achieved R0 resections after neoadjuvant treatment with a pCR rate of 15%.Median PFS and OS were 15.1 mo and 30.5 mo,respectively[72].
Chromosomally instable (CIN) tumors are the most frequent,accounting for around 50% of GCs and they usually appear at the GEJ.These tumors display marked aneuploidy and have a considerable number of genomic amplifications of key receptor tyrosine kinases,cell cycle regulation genes and transcription factors.They are associated with intestinal histology and most tumors carrymutations and RTKRAS activation[19].Prognosis is similar to MSI subtype,however,CIN subtype seems to receive the largest benefit from adjuvant chemotherapy[17].
He flew into a dreadful rage when he saw them, and screamed out, Oh, you fools! the river and bridge were they! Go back and bring them to me at once, or it will be the worse for you
GS subtype lacks the molecular characteristics of the other three subtypes and has tumors enriched for the diffuse histologic variant,with approximately 30% of them having mutations or fusions in the CDH1 and RHOA signaling pathway.This group accounts for 20% of GCs that are characterized by the lack of high levels of aneuploidy and high metastatic potential.It carries the worst prognosis of all the subtypes and receives little benefit from adjuvant chemotherapy,according to Sohn’s model[17].CDH1 germline mutations are usually associated with diffuse GC hereditary syndrome[7].
The TCGA project examined GEJ tumors separately in the molecular analysis of esophageal carcinoma[9].Study results concluded that adenocarcinomas of the esophagus,including GEJ,more closely resemble GC,especially of the CIN subtype.GEJ tumors are characterized by DNA hypermethylation and while most tumors are classified to the CIN subtype,EBV or MSIH positivity is not uncommon[9].
TREATMENT
Curative treatment of GC can be achieved at the early stage through surgical or endoscopic resection.Other treatment modalities,such as radiation and chemotherapy,are frequently employed to increase the chances of successful resection and prevent distant relapse.Several studies have explored this combination of modalities and the ideal sequencing is yet unclear.
Role of preoperative chemotherapy
Preoperative chemotherapy is being employed in many different solid tumors as it offers certain advantages over postoperative therapy.Preoperative chemotherapy has a chance of reducing tumor size and facilitates surgical excision with negative margins(R0 resection)[20].Furthermore,patients have a higher chance of completing preoperative chemotherapy rather than postoperative,due to possible postoperative complications or decrease in performance status associated with gastrectomy-related comorbidities.Prevention of distant metastases until surgery is also an important goal of preoperative chemotherapy[21].Finally,response to preoperative chemotherapy has been shown to have prognostic value in patients with breast and rectal cancer,with patients achieving pathologic complete response (pCR) enjoying longer diseasefree survival (DFS)[22].Newer retrospective data also suggested a similar pattern for gastric and GEJ cancer[23,24],while results from the prospective trials cited below showed a trend for improved outcomes in patients achieving complete response in preoperative therapy.
Principles of surgical excision
Results from the TOPGEAR study[61] are also awaited.TOPGEAR is an ongoing international phase III trial in patients with adenocarcinoma of the stomach and GEJ receiving induction perioperative chemotherapy with epirubicin/cisplatin/5-FU (ECF)alone or in combination with preoperative chemoradiation.The ECF group receives three preoperative cycles of ECF,while the chemoradiation group receives two cycles of ECF followed by chemoradiation.After surgical excision,patients in both groups are receiving three cycles of ECF.An interim analysis of 120 recruited patients indicated that 90% in the ECF group and 85% in the chemoradiotherapy group underwent gastrectomy.Results on effectiveness and comparison among groups are pending[61].
Role of radiotherapy
Radiation therapy has been employed as a mean of local tumor control in most solid tumors,including gastric cancer.Ionizing radiation targets cells during their proliferative phase,and tumor cells are more susceptible to radiation damage than regular tissue cells[28].Radiotherapy may be used with a curative or palliative intent,depending on the total dose received and the urgency for local tumor control.In metastatic gastric and gastroesophageal cancer,radiation has been used to alleviate symptoms such as bleeding,pain and obstruction with variable but generally satisfying results in different observation studies[29].In the early setting,radiation is being used concomitantly with chemotherapy to achieve better tumor control before or after surgical excision.Whether the addition or the timing of radiation therapy to standard chemotherapy offers additional clinical benefits remains the question of ongoing clinical trials which will be further discussed in this paper.
Combination of chemotherapy and radiotherapy
Chemotherapy is being used concomitantly or sequentially with radiotherapy in the treatment of many localized solid tumors.Definitive chemoradiation is the modality of choice in locally advanced head and neck cancers,lung and rectal cancers[30].Apart from the separate cytotoxic effects of each type of treatment,concurrent use of chemotherapy and radiotherapy has shown to produce a synergistic effect that enhances antitumor response,which is more apparent than when used sequentially[31].Chemotherapy acts as a radiosensitizer,probably by allowing cells to inappropriately progress through the S phase of the cell cycle,thus not permitting to repair the DNA damage caused by radiation[32].Furthermore,combination treatment modulates tumor microenvironment[33],and allows for simultaneous control of both systemic micrometastases and local disease.Radiation and chemotherapy combination has been thoroughly studied and has solidified its role in the treatment of gastric and esophageal cancer,particularly in locally advanced SCC of the esophagus,where disease control can be achieved with chemoradiation alone and surgery can be reserved for refractory or relapsed disease[34].
Addition of immunotherapy
Recently,with the increasing use of checkpoint inhibitors,significant interaction has been noted between immunotherapy and radiotherapy that may lead to increased antitumor response.One possible mechanism to explain this effect is the release of tumor neoantigens in the tumor microenvironment after cellular destruction due to radiation.Exposure of stromal immune cells to tumor neoantigens may induce an antitumor response,further enhanced by the presence of immune checkpoint inhibitors[35].Several preclinical data support this notion.For example,a study by Deng[36] in mouse models of breast and colorectal cancer showed that combination of radiation and PD-L1 inhibitors,controlled tumor growth more effectively than radiation or immunotherapy alone (587.3 ± 169.1 mm with anti–PD-L1 alone25.59 ±10.26 mm with radiation plus anti–PD-L1=0.0022,402.8 ± 76.73 mm with radiation alone vs.25.59 ± 10.26 mm with radiation plus anti–PD-L1,=0.0002).
Addition of radiotherapy to chemotherapy after gastrectomy has been explored in several trials (Table 1).A classic study by Macdonald[37] examined the effectiveness of adding postoperative chemoradiotherapy to surgical resection in patients with adenocarcinoma of the stomach and GEJ.The trial included a total of 556 patients which were randomized to receive surgery alone or surgery with postoperative chemoradiotherapy.Most patients (67%) had T3 disease and positive lymph nodes (84%).Gastric antrum was the most common primary tumor location.However,more than half of the patients underwent suboptimal lymph node dissection (54% D0,36% D1,10% D2).Chemotherapy consisted of 5-fluorouracil (5-FU) and leucovorin and was administered concurrently with radiation.Addition of chemoradiotherapy increased OS to 36 mo,in comparison to 27 mo in the surgery-only arm,in a statistically significant manner.Grade 3 adverse events were more common in patients in the chemoradiotherapy arm[37].
8. Terribly dark, and raining so heavily and blowing so hard: In other words, it was a dark and stormy night, the now cliched setting for a story.Return to place in story.
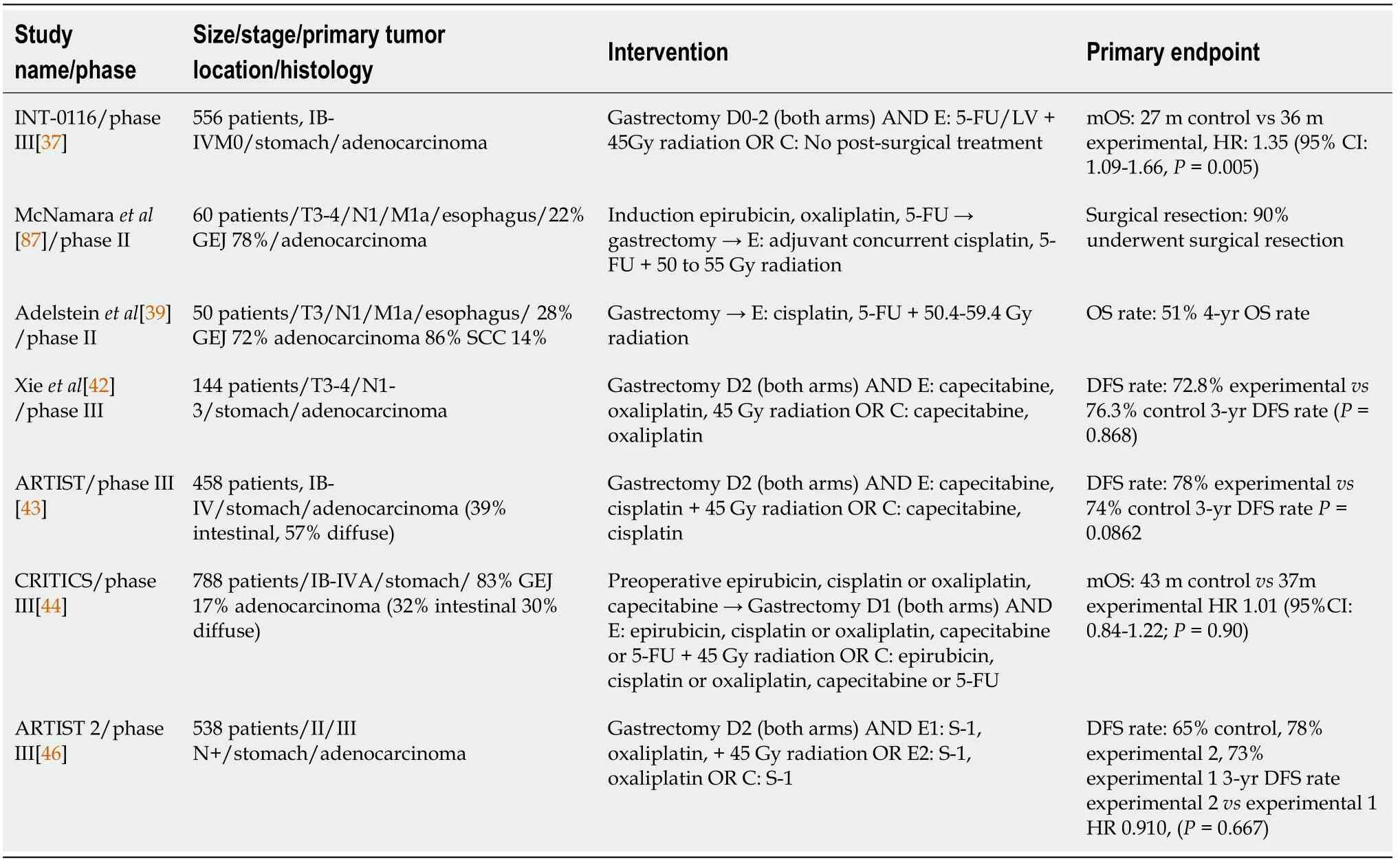
Similarly,a phase II study by McNamara[38] tested the effectiveness of adjuvant chemoradiotherapy with cisplatin and 5-FU after neoadjuvant chemotherapy with epirubicin,oxaliplatin and 5-FU,in patients with resectable adenocarcinoma of the esophagus and GEJ.60 patients were recruited and GEJ was the most common primary tumor location.An objective response was achieved in 41% of the patients and a pCR in 5% of them.The 3-year locoregional control rate reached 84% and distant metastasis control rate was 44%,while total relapse free survival was projected at 39%.3-year OS rate was calculated at 42%.Clinical response to induction therapy was strongly associated with better outcomes[38].
Another small phase II study by Adelstein[39] examined the effectiveness of postoperative chemoradiotherapy in patients with locally advanced tumors of the esophagus and GEJ.The study enrolled 50 patients in total.Among them,43 patients were diagnosed with adenocarcinoma,36 had a tumor located in the GEJ and 86% of patients had node-positive disease.The study also included 3 patients with M1a disease.In the overall study population,the 4-year projected OS was 51%,freedom from recurrence was 50% and distant metastases control rate was 56%.No major difference was observed among different patient subgroups,apart from disease stage and a marginal benefit for patients with SCC in comparison to adenocarcinoma[39].
But they were not very, very far from land, and there was just enough strength left in the North Wind to enable him to throw her on to the shore, immediately under the windows of a castle which lay east of the sun and west of the moon; but then he was so weary and worn out that he was forced to rest for several days before he could go to his own home again
A retrospective pooled analysis by Dikken[40] evaluated the effectiveness of adjuvant chemoradiotherapy in comparison to surgical excision alone in 91 patients with GC from two phase I/II studies and 694 patients from the Dutch Gastric Cancer Group Trial (DGCT).Patients in the DGCT group underwent only surgery and were randomly assigned to D1 or D2 Lymph-node dissection.Patients in the phase I/II trials received chemotherapy with 5-FU and leucovorin,capecitabine alone or capecitabine with cisplatin.Local recurrence at 2 years was significantly higher in the surgery-only group (17%5%) in the overall study population.Subgroup analysis according to the extent of lymph node dissection showed a statistically significantly lower recurrence rate in the chemoradiation arm in the D1 subgroup (2%8%);however,no difference was observed in patients that had D2 surgery.Chemoradiation also improved outcomes of patients that underwent surgical excision with microscopically positive margins (R1 resection)[40].
We went to a restaurant that, although not elegant2, was very nice and cozy3() . My mother took my arm as if she were the First Lady. After we sat down, I had to read the menu. Her eyes could only read large print. Half way through the entries, I lifted my eyes and saw Mom sitting there staring at me. A nostalgic smile was on her lips.
A recent Turkish retrospective analysis of 354 patients with resectable GC associated postoperative chemoradiation with improved relapse free survival (RFS),albeit the percentage of patients that underwent D2 lymph node dissection is unclear.Median RFS in the whole study population reached 53.2 mo and median OS 136 mo.Interestingly,another factor associated with an increased risk for relapse was preoperative hypoalbuminemia[41].
More recent trials,using optimized surgical techniques,have presented interesting data on the benefit of adjuvant chemoradiotherapy.A phase III trial by Xie[42] in 144 patients with locally advanced GC,staged as T3-4/N1-3 were randomized to receive either adjuvant capecitabine plus oxaliplatin or the same regimen with the addition of radiotherapy,after completion of D2 gastrectomy.3-year disease-free survival did not differ significantly between arms,with 76.3% in the chemotherapy arm and 72.8% in the chemoradiation arm.A similar pattern was noted for OS.Rate of local RFS was also similar between arms,with no added benefit seen from the addition of radiotherapy.Similarly,no difference was noted in DFS in patients with lymphnode positive disease[42].
I n a similar manner,the ARTIST study[43] compared the effectiveness of adding radiotherapy to adjuvant chemotherapy in prolonging DFS and OS in patients with GC.Investigators recruited 458 patients with GC that underwent gastrectomy with D2 Lymph node dissection.Over 80% of patients in each cohort had positive lymph nodes and the majority of patients had stage II and III disease.Most common location of the primary tumor was the body of the stomach.Patients were also stratified according to Lauren classification and around 60% in each arm belonged to the diffuse subtype.Patients were randomly assigned to either adjuvant chemotherapy with six cycles of capecitabine and cisplatin or to two cycles of capecitabine and cisplatin followed by chemoradiotherapy and two cycles of cisplatin and capecitabine after completion.DFS and OS showed no statistically significant difference among the two cohorts in the study’s overall population.The only subgroups that seemed to derive a statistically significant benefit in DFS and OS from the addition of radiotherapy were patients with lymph node positive disease and intestinal subtype.Patients with stage III or IV disease showed a trend towards improvement,without reaching statistical significance[43].
Another study pointing to the same direction was CRITICS phase III study[44],in which 788 patients with gastric and GEJ adenocarcinomas were enrolled and randomized to receive either adjuvant chemotherapy with combination of epirubicin,cisplatin or oxaliplatin and capecitabine or adjuvant radiation therapy concurrently with cisplatin and capecitabine.All patients received preoperative chemotherapy and 741 patients underwent gastrectomy with at least D1+ lymph node dissection.Of them,478 patients received adjuvant therapy in their respective cohorts.Lauren histologic subtypes were equally represented within each cohort and only 17% of patients in each cohort had cancer of GEJ.Almost half of the patients in each cohort had node-negative disease after gastrectomy.Median OS was higher in the chemotherapy groupthe chemoradiotherapy group (43 mo37 mo) although this difference did not reach statistical significance in any subgroup[44].
The recent ARTIST 2 trial[45] evaluated the addition of oxaliplatin or oxaliplatin and radiotherapy to adjuvant treatment of patients with stage II or III GC who underwent gastrectomy and D2 Lymph node dissection with positive lymph nodes.538 patients were randomized into three cohorts according to the type of adjuvant treatment and were stratified according to the type of surgery,stage,and Lauren subtype.Patients in the first cohort received adjuvant S-1,in the second S-1 plus oxaliplatin and S-1 plus oxaliplatin and chemoradiotherapy in the third cohort.DFS was significantly lower in the S-1 only arm in comparison to S-1 plus oxaliplatin and S-1 plus oxaliplatin and chemoradiation,while there was no statistically significant benefit with the addition of radiotherapy.Interim results met the pre-specified endpoints sufficiently and the trial was terminated earlier than planned[46].
Postoperative chemoradiotherapy has improved local control rates and improved disease-free survival in earlier studies before adjuvant chemotherapy became standard of care in gastric and GEJ cancer.Although certain benefit in local control might exist for patients with less than D2 Lymphadenectomy,results from randomized phase III CRITICS and ARTIST 2 studies showed no additional benefit in clinical outcomes in comparison to adjuvant chemotherapy.
The benefit of adding radiotherapy to preoperative chemotherapy in the management of GC has been a topic of debate,with many highly heterogeneous studies reporting conflicting results.Several small studies have been conducted,evaluating feasibility and effectiveness (Table 2).

Analysis of recent real-world data can also add to the existing knowledge on the management of operable gastroesophageal cancer.A study of 1916 patients from the nationwide Netherlands Cancer Registry (NCR)[62],with esophageal or GEJ cancer undergoing curative treatment,with surgery or definitive chemoradiation,reported on real-world treatment outcomes.The majority of patients underwent surgery and only 21% received definitive chemoradiation.Out of patients with resected disease,83%underwent neoadjuvant chemoradiation and 10% neoadjuvant chemotherapy,with or without adjuvant chemoradiation.Only 7% received surgery alone.Patients that received definitive chemoradiation had shorter median DFS (14.2 mo26.4 mo) and median OS (20.9 mo40.5 mo) than patients that underwent surgical resection.However,median age was higher and performance status was worse in the definitive chemoradiation group.Patients that received neoadjuvant chemoradiation had a median DFS of 25.2 mo and a median OS of 38 mo.Interestingly,this study included a separate subgroup analysis of patients that received adjuvant nivolumab after trimodality treatment with chemoradiation and surgery,as part of CheckMate-577 trial.Among these patients the median DFS and median OS were 19.2 and 29.4 mo,respectively[62].
The phase II RTOG 9904[48] study evaluated the effectiveness of neoadjuvant chemoradiotherapy in patients with resectable GC.49 patients were enrolled and received preoperative chemotherapy with cisplatin,5-FU and leucovorin concurrently with radiation,followed by surgery.The majority of patients had stage III disease.pCR and R0 rates reached 26% and 77% respectively and pCR was associated with favorable prognosis,in accordance with other previous studies.D2 resection was performed in only 50% of the patients[48].
The little tailor continued to follow his nose, and after he had wandered about for a long time he came to the courtyard of a royal palace, and feeling tired he lay down on the grass and fell asleep
A few other small studies have evaluated trimodality treatment in patients with esophageal and GEJ carcinomas.The phase II S0356 study[49] explored the impact of neoadjuvant chemoradiotherapy in patients with clinical stage II-III esophageal and GEJ adenocarcinomas.The study enrolled 93 patients,including 36 patients with adenocarcinomas of GEJ,who received a neoadjuvant combination of oxaliplatin and 5-FU and radiotherapy followed by surgical excision.After surgery,patients were planned to receive adjuvant chemotherapy with oxaliplatin and 5-FU.Genomic analysis of DNA and mRNA was also performed,seeking potential new prognostic and predictive biomarkers.The primary objective of this study was to achieve a pCR rate of 40%.79 patients underwent surgery and 67.7% achieved R0 resections,26 patients (28%) achieved pCR,thus not reaching the pre-specified endpoint of 40% and estimated median OS and 3-year OS were 28.3 mo and 45.1%,respectively.In terms of genomic analysis,ERCC-1 gene expression was associated with worse PFS and OS[49].
A similar phase II study by Ilson[50] evaluated the effectiveness of preoperative chemoradiation in patients with esophageal and GEJ carcinoma.The study included 55 patients in total,with both squamous (22%) and adenocarcinoma (75%) histologies.Primary tumor location was the GEJ in 33% of the patients.Patients received induction chemotherapy with combination of cisplatin and irinotecan,followed by concurrent chemoradiation.Out of them,16% achieved pCR and median OS reached 31.7 mo.Patients were also evaluated for correlation between positron emission tomography(PET) response to induction chemotherapy and pCR rate,R0 resection rate,PFS and OS.PET response was significantly associated with higher pCR rate,PFS and chance of R0 resection.OS was increased in PET-responders,although not in a statistically significant manner[50].Due to positive results from the addition of radiotherapy to neoadjuvant chemotherapy from single arm trials,direct comparison with a chemotherapy or surgery only approach has been employed in smaller phase II studies and paved the way for larger phase III trials.
A phase II randomized trial on the effectiveness of trimodality treatment by Ajani[51] randomized 126 patients with esophageal and GEJ carcinoma to neoadjuvant chemoradiotherapy with or without induction chemotherapy with oxaliplatin and 5-FU,followed by surgical resection.122 patients (96.8%) were diagnosed with adenocarcinoma and 122 patients (96.8%) had tumors located in the GEJ.Median OS was 45.6 mo for all patients,with median OS being 45.6 mo in the no-induction arm and 43.6 mo in the induction arm,with this difference not reaching statistical significance.The pCR rate was numerically higher in the induction chemotherapy arm(26%13%)[51].
If the boy had seen her he would have been changed into stone by the terror and the pity of it, she was so awful; but he had thought of a plan for killing30 her without looking on her face
Smaller studies have investigated anti-EGFR antibody cetuximab and anti-VEGF antibody bevacizumab in combination with chemoradiotherapy and surgery.In the phase III SAKK 75/08 trial[71],300 patients with esophageal cancer were randomly assigned to receive neoadjuvant chemoradiation with or without the anti-EGFR antibody cetuximab,followed by surgical resection.More than half of patients (63%)were diagnosed with adenocarcinoma.Chemoradiotherapy consisted of cisplatin and docetaxel and radiation of 45 Gy.Cetuximab was given throughout chemoradiation and as adjuvant monotherapy after surgery.The study did not meet its primary endpoint,which was a statistically significant improvement in PFS [2.9 years in cetuximab arm2 years in control arm (HR 0.79,95%CI:0.58-1.07;=0.13)].Median OS was numerically improved (5.1 years3 years) but this difference did not reach statistical significance.Similarly,subgroup analysis did not show any advantage with the addition of cetuximab,regardless of histologic type.However,locoregional control was significantly better in the cetuximab arm[71].
Quite true, answered Death; but his foot is in my kingdom, and that belongs to me! At any rate half of him is mine, replied the Queen, and what good can the other half do you? Half a man is no use, either to you or to me! But this once I will allow you to cross into my kingdom, and we will decide by a wager41 whose he is
Accordingly,the NeoRes study[53] conducted in Norway and Sweden recruited 181 patients with malignant tumors of the esophagus and GEJ.The most prevalent histologic type was adenocarcinoma (72% of patients) and 17% of tumors were located in the GEJ.Patients were equally randomized to receive neoadjuvant chemotherapy alone or with the addition of radiotherapy.The pCR rate was higher in the chemoradiation arm and lymph node positivity was lower at the time of surgery.OS did not differ between the two arms.Later results confirm that the addition of radiation to neoadjuvant chemotherapy did not significantly affect 5-year PFS and OS[53,54].
Despite the large number of trials addressing this topic,conclusions cannot be clearly drawn,due to a large number of confounding factors,low adherence to treatment protocols and high group heterogeneity.A meta-analysis by Zhao[55] of six clinical trials,including the ones by Stahl and Klevebro,that included 866 patients with adenocarcinoma and SCC of the esophagus and GEJ,concluded that 3-year and 5-year OS rates were improved with the addition of radiotherapy to neoadjuvant chemotherapy in a statistically significant manner.Furthermore,neoadjuvant chemoradiotherapy increased the chance of R0 resection and pCR.This benefit seems to apply to patients with both adenocarcinomas and SCCs[55].
Larger phase III trials have attempted to produce more robust evidence and provide definitive answers on the benefit of trimodality treatment.A small phase III trial CALGB 9781[56] evaluated the use of trimodality treatment with chemotherapy including cisplatin,5-FU and radiotherapy before surgical resection of esophageal or GEJ carcinomas.The trial was terminated early due to poor accrual and only 56 patients were evaluable for response.23 patients in the chemoradiotherapy cohort and 19 in the surgery-alone cohort had adenocarcinomas (77% and 73%,respectively).In the overall study population,median OS reached 4.48 years in comparison to 1.79 years with surgery-alone arm.However,the final population study was small,and no further data on different subgroups were evaluable[56].
The largest dataset from a randomized clinical trial currently available is from the CROSS phase III trial[57],which evaluated the effectiveness of neoadjuvant chemoradiotherapy in patients with resectable esophageal and GEJ carcinomas.366 patients were randomly assigned to receive either combination of weekly carboplatin and paclitaxel with concurrent radiotherapy over a 5-wk period and then proceed to surgical resection,or to surgery-alone.The study included patients with different histologies,with the majority being adenocarcinomas (75%).In 88 patients,the primary tumor location was GEJ.Around 65% of patients in each cohort had lymphnode positive disease.Patients in the chemoradiotherapy group achieved a statistically significant higher degree of R0 resections in comparison to surgery alone (92%69%),while 29% in the chemoradiotherapy group achieved pCR at the end of neoadjuvant treatment.Postoperative complications were similar between the two subgroups,and OS was significantly improved in the chemoradiotherapy group (49.4 mo24.0 mo).In subgroup analysis,patients with node-negative disease at diagnosis and patients with squamous histology received clear benefit,while patients with adenocarcinoma showed a clear trend towards improvement[57].
Although CROSS study[44] proved that neoadjuvant chemoradiotherapy leads to improved outcomes in comparison to surgery alone,data from MAGIC trial[58] show also improved OS with preoperative chemotherapy,while FLOT4 trial[59] identified FLOT as a superior regimen.Thus,the ideal neoadjuvant approach is still unclear and the benefit of trimodality therapy is still under discussion.
“The bright warm sun shone on a little court, on the first warm day of spring. His bright beams rested on the white walls of the neighboring house; and close by bloomed the first yellow flower of the season, glittering like gold in the sun’s warm ray. An old woman sat in her arm chair at the house door, and her granddaughter, a poor and pretty servant-maid came to see her for a short visit. When she kissed her grandmother there was gold everywhere: the gold of the heart in that holy kiss; it was a golden morning; there was gold in the beaming sunlight, gold in the leaves of the lowly flower, and on the lips of the maiden. There, that is my story,” said the buttercup.
As mentioned,there is an adequate amount of evidence on the addition of radiation therapy to chemotherapy in resectable gastric and GEJ cancer.However,as mentioned in the studies above,it is clear that there is heterogeneity concerning the chemotherapy regimen used.Several efforts have been made to identify the ideal chemotherapy regimen to partner with preoperative or postoperative radiation (Table 4).
Soon he saw the golden mermaid swimming near the ship, beckoning33 and calling to him to follow her; but, mindful of the wolf s warning, he told her in a loud voice that if she wished to buy anything she must come to him
Adenocarcinomas of the GEJ can be classified according to the Siewert classification,into type I,which arise from the distal esophagus,type II which is true junctional carcinoma of the cardia/esophagus and type III,which is subcardial carcinoma may invade the GEJ from below[25].The extent of gastrectomy as well as the reconstruction technique used depends on tumor location.Gastrectomy is accompanied by lymph node dissection,which can be classified as D1,D2 or D3 dissection.D1 Lymphadenectomy includes all N1 nodes (perigastric nodes) whereas D2 Lymphadenectomy consists of removal of both N1 and N2 nodal groups (distant perigastric nodes and nodes along main arteries supplying the stomach)[26].D3 Lymphadenectomy is an extensive resection including N3 nodes (para-aortic lymph nodes).D2 resection has been proved to have an advantage in improving OS,however D3 dissection failed to show any benefit in comparison to D2,and is associated with increased postoperative morbidity[27].
A phase II study by Rivera[47] aimed to determine the benefit of adding chemoradiotherapy to preoperative chemotherapy with irinotecan and cisplatin in 23 patients with resectable,locally advanced,stage II-IV adenocarcinoma of the stomach and GEJ.Patients received two courses of irinotecan and cisplatin followed by irinotecan and cisplatin plus external beam radiation.In patients without progression,surgical resection was performed.Among the evaluable patients,2 achieved pCR.Median OS was 14.5 mo and 2-year OS rate reached 35%[47].
Another retrospective study by Spencer[63] evaluated the role of neoadjuvant chemoradiation in patients with stage III or IVA locally advanced (T3/T4) adenocarcinoma of the esophagus and GEJ,followed by surgical resection.Patients with threatened circumferential resection margin by imaging received neoadjuvant chemoradiation,while those without a threatened margin received neoadjuvant chemotherapy.Patients that received neoadjuvant radiation also received a combination of carboplatin and paclitaxel.Most patients in the chemotherapy group received a platinum-fluoropyrimidine doublet.In total,results from 81 patients were reported.18 patients received chemoradiation and 63 patients chemotherapy alone.Both groups included 5 patients with stage IVA disease.Rates of R0 resection were higher in the chemoradiation group and rate of local relapse was lower in comparison with the chemotherapy group.However,no difference was noted in OS and RFS.R1 resection in the chemotherapy group was a negative prognostic factor[63].
Jurkowski[64] reported on the outcomes of patients with locally advanced esophageal and GEJ adenocarcinomas treated with total neoadjuvant therapy,including induction chemotherapy followed by chemoradiation.Induction chemotherapy included doublet or triplet regimens of 5-FU,cisplatin or oxaliplatin and docetaxel,while carboplatin and paclitaxel,or oxaliplatin and 5-FU were used concurrently with radiation.37 out of 59 evaluable patients underwent surgical resection.9 patients opted out of surgery since they achieved clinical complete response.Among the patients who received surgery,R0 rate was 89% and pCR rate was 19%.For the entire population of the study,median DFS was 2.4 years and median OS 4.7 years.Patients who underwent surgery had a higher DFS and median OS (3.5 years1.5 years and 5.8 years4.2 years).The subgroup that achieved clinical complete response had worse 3-year DFS in comparison to operated patients with pCR (42%83%),however 3-year OS was improved (89%83%)[64].
Preoperative radiation is frequently employed in patients with gastroesophageal cancer and represents the standard of care in some many high-volume centres.Prospective and retrospective date suggest a role for preoperative chemoradiation in improving local tumor control and achieving higher pCR rates.Whether this translates to long-standing improvement in overall survival remains the subject of ongoing clinical trials,such as TOPGEAR and Neo-AEGIS.While Neo-AEGIS showed noninferiority of preoperative chemotherapy to the CROSS regimen,it is unclear whether addition of chemoradiation to induction chemotherapy would maximize clinical benefit until results from the TOPGEAR study are announced.Moreover,only 27 of 184 patients in the chemotherapy-alone arm received FLOT,which has proved to be superior to the MAGIC regimen[59].
Combination with newer therapeutic agents
Newer treatment modalities have been constantly added to the therapeutic arsenal in metastatic gastroesophageal cancer.Targeting agents,such as anti-HER2 antibodies,and immune checkpoint inhibitors,as well as new predictive and prognostic biomarkers have all prolonged survival and improved quality of life in patients with unresectable disease[65].Several modalities are being investigated in the early setting and some have already produced encouraging results (Table 3).

The first trial of immunotherapy to produce positive results in early gastroesophageal cancer is CheckMate 577[66].It is a global,multi-center,randomized,double-blind phase III study that explores the addition of anti-PD1 checkpoint inhibitor nivolumab to trimodality treatment for esophageal and GEJ carcinoma.794 patients with resected,stage II/III,esophageal/GEJ carcinoma who received neoadjuvant chemoradiation and did not achieve a pCR were randomized 2:1 to receive nivolumab 240 mg every 2 wk for 16 wk,followed by 480 mg every 4 wk up to 1 year or placebo.Most dominant histology was adenocarcinoma(71%) and most patients had positive lymph nodes (60%).The primary endpoint of the study was median DFS,which was doubled with the addition of nivolumab in comparison to placebo (22.4 mo11.0 mo).Severe treatment-related adverse events(TRAEs) occurred in 8% of nivolumab patients and 3% of placebo patients.The most common TRAEs were fatigue,pruritus,diarrhea and rash.Grade 3 immune related adverse events occurred in less than 1% of patients in the nivolumab arm[66].
Apart from the proven benefit of nivolumab,a newer phase II[67] study is examining the role of adding durvalumab to neoadjuvant chemoradiotherapy in patients with esophageal and GEJ adenocarcinoma.Study design was based on the results of CALGB 80803[68],a study that evaluated response to induction chemotherapy by PET/computed tomography (CT).After 2 cycles of mFOLFOX6,PET responders received chemoradiation with capecitabine and oxaliplatin and radiation to 50.4 Gy,while PET non-responders switched to different chemotherapy regimen of carboplatin/paclitaxel concurrent with radiation.Durvalumab was added to all patients,2 wk before chemoradiation and was continued during chemoradiation,and was continued after R0 resection.According to preliminary data,36 patients have been recruited,25 with adenocarcinoma of GEJ and 11 with adenocarcinoma of the esophagus.Out of them,72% showed disease response to induction chemotherapy at PET/CT.25 patients underwent surgical resection and 6 achieved pCR,while 5 patients were downstaged to ypT1N0 and 2 patients to ypT0N1,showing 99%response.Another 20 patients had more than 90% response.Grade 3/4 neutropenia was observed in 8 patients and grade 3 hepatitis in 1 patient.More data are still pending[67].
Immunotherapy has gained importance in gastric and gastroesophageal cancer due to recent results from first-line phase III studies (Checkmate 649,Keynote 570)showing efficacy in the metastatic setting.As a result,combination of chemotherapy and immunotherapy is being used earlier in the therapeutic algorithm.CheckMate 577 is the first study to prove the benefit of adding postoperative immunotherapy in patients with gastroesophageal cancer and residual disease following preoperative chemoradiation.Ongoing and future studies will address the question of incorporating immunotherapy in trimodality treatment of gastric cancer,either concurrently or sequentially with chemotherapy and/or radiation therapy.
Given the effectiveness of anti-HER2 antibody trastuzumab in the management of advanced HER2 overexpressing GC,several trials have evaluated the benefit of HER2 inhibition in the early setting in combination with trimodality treatment.
The phase III RTOG 1010 trial[69] evaluated the effectiveness of adding the anti-HER2 agent trastuzumab concurrently with chemoradiation in patients with resectable,HER2 overexpressing (determined by immunohistochemistry and fluorescence in situ hybridization) adenocarcinoma of the esophagus and GEJ.Chemoradiotherapy consisted of carboplatin and paclitaxel and radiation of 50.4 Gy of 3D-chemoradiation therapy or intensity modulated radiotherapy.Trastuzumab was administered throughout the chemoradiation period and for 13 more cycles after surgery.In total,203 patients were randomized to receive neoadjuvant chemoradiotherapy with or without trastuzumab.The study did not reach its primary objective,which was statistically significant improvement in DFS.Median DFS was 19.6 mo in the experimental arm and 14.2 mo in the control arm (HR 0.97,95%CI:0.69-1.36).Median OS also did not differ between the two arms[69].
The phase II TOXAG study[70] also evaluates the safety and efficacy of adding trastuzumab to neoadjuvant chemoradiation with oxaliplatin,capecitabine and radiation in patients with HER2+ adenocarcinoma of the stomach and GEJ who will undergo curative surgical resection.212 patients have been screened and 34 underwent surgical resection.The combination regimen of oxaliplatin,capecitabine,trastuzumab and radiation has achieved a high rate of tolerability of 90.3% and 97% of patients achieved D2 Lymph node dissection.At 25 mo follow-up,59.8% of patients were still alive,and 12 patients have died because of disease progression.More data on effectiveness of treatment are still pending[70].
Similarly,the POET trial[52] recruited 119 patients with locally advanced GEJ adenocarcinoma (Siewert types I-III) and randomized them to receive either chemotherapy with cisplatin and 5-FU alone or chemotherapy and chemoradiation.Patients in both cohorts were treated with surgical resection afterwards.Local PFS after tumor resection was significantly improved in the chemoradiation arm and there was a trend towards improvement of OS,without reaching the pre-specified endpoint for statistical significance[52].
Cherry-scented smoke from Grampy s pipe kept the hungry mosquitoes at bay while gray, wispy2(,) swirls3 danced around our heads. Now and again, he blew a smoke ring and laughed as I tried to target the hole with my finger. I, clad() in a cool summer nightie, and Grampy, his sleeveless() T-shirt, sat watching the traffic. We counted cars and tried to guess the color of the next one to turn the corner.
Anti-HER2 and antiangiogenic agents have improved clinical outcomes in patients with metastatic gastric and gastroesophageal cancer.However,in the early setting,targeted agents combined with standard of care have not yet produced satisfactory results.The phase III RTOG 1010 of trastuzumab plus chemoradiation has failed to prove additional clinical benefit with the addition of trastuzumab.The anti-EGFR agent cetuximab did not improve PFS in the phase III SAKK 75/08 in comparison to standard chemoradiotherapy alone.Comparative data are not yet available on the use of anti-VEGF agents.
Choice of chemotherapy regimen
Recent results from the phase III Neo-AEGIS are the first comparative data available answering this question.Neo-AEGIS is a phase III randomized European study comparing the efficacy and safety of preoperative chemoradiation,per CROSS study protocol,to chemotherapy alone,per MAGIC or FLOT4 protocol in patients with resectable esophageal and GEJ adenocarcinomas[60].The study initially attempted to prove superiority of the CROSS regimen over chemotherapy,however after the first futility analysis a non-inferiority approach was adopted.Chemotherapy alone reached the primary endpoint of non-inferiority in terms of 3-year OS [57% for chemotherapy56% for chemoradiation hazard ratio (HR) 1.02 (95%CI:0.74-1.42)].Of note,more patients in the CROSS arm achieved pCR and significant tumor shrinkage,in accordance with results from earlier clinical trials showing improved tumor local control with preoperative chemoradiation[60].
A retrospective study by Jiang DM[73] compared carboplatin plus paclitaxel with cisplatin plus fluorouracil as chemotherapy regimens in patients with resectable tumors of the esophagus and GEJ that received neoadjuvant chemoradiotherapy.The study also included patients who did not proceed to surgical resection and received definitive chemoradiation.93 patients with esophageal (49%) and GEJ (51%) tumors that received neoadjuvant (72%) or definitive (28%) chemoradiation were identified.53 patients had received cisplatin-5-FU and 40 carboplatin-paclitaxel.59 patients were diagnosed with adenocarcinoma and 34 with SCC.In patients who received surgery,no difference was observed between the two treatment groups.However,in the definitive chemoradiation subgroup,carboplatin-paclitaxel was associated with significantly worse 3-year OS (36%63%=0.001 HR 3.1,95%CI:1.2-7.7) and DFS(0%41%;=0.004;HR 3.6,95%CI:1.4-8.9)[73].
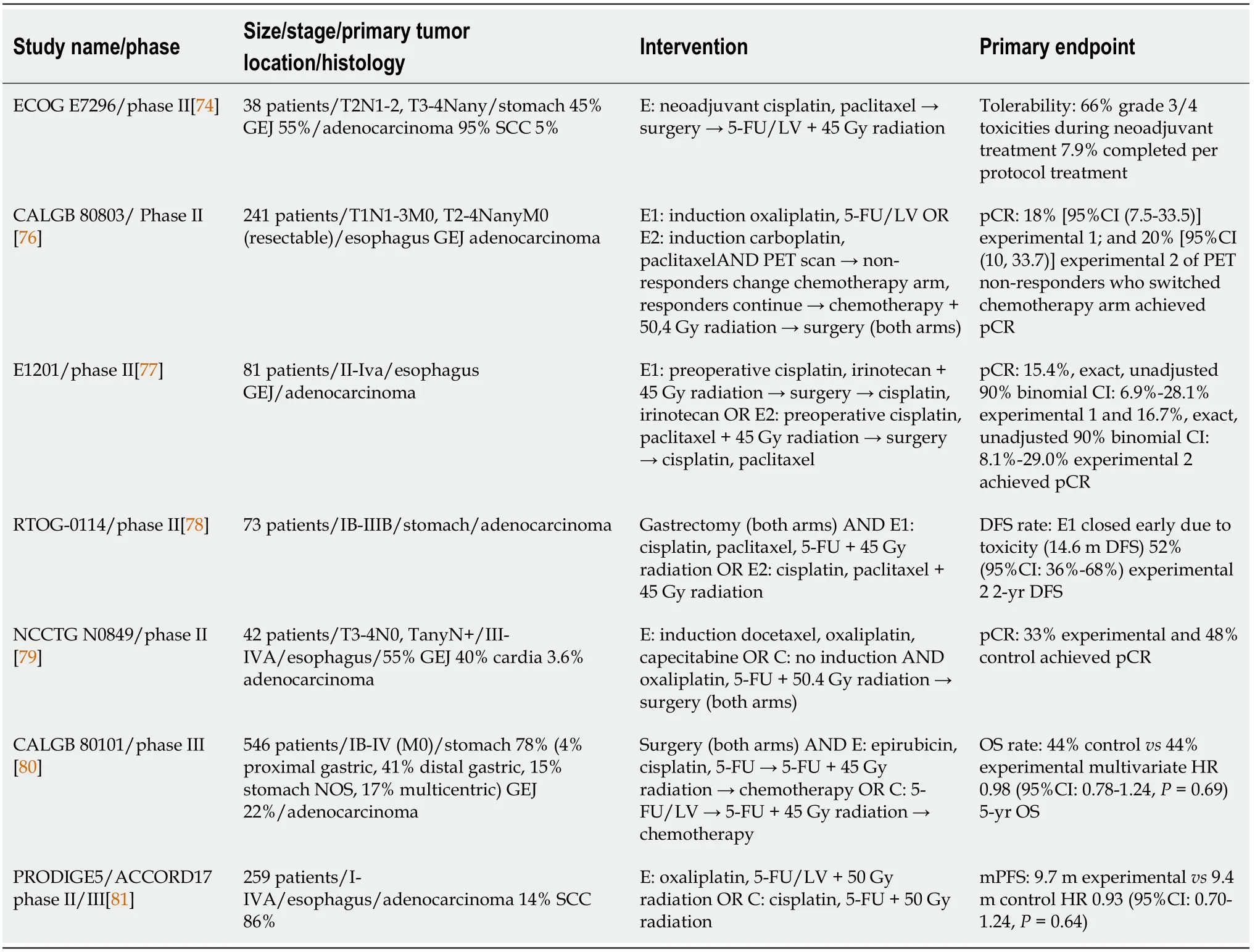
In the phase II,ECOG E7296 study[74],patients with carcinomas of the stomach and GEJ received adjuvant chemoradiation after induction chemotherapy and surgical resection.38 patients were enrolled and among them,36 were diagnosed with adenocarcinoma and 21 with tumor of the GEJ.Induction chemotherapy consisted of cisplatin and paclitaxel,while adjuvant chemotherapy of leucovorin and 5-FU.No pCR was noted after induction chemotherapy and only 3 out of 38 patients completed treatment per protocol design.Median OS in the overall population was 1.6 years.The regimen used in this study was evaluated to be highly toxic and,thus,further development was discouraged by the investigators[74].
Another study,CALGB 80803,a phase II study[68] attempted to individualize chemotherapy in patients with resectable esophageal and GEJ adenocarcinomas receiving preoperative chemoradiation,by using early PET/CT scan.241 patients were enrolled and were randomized to receive induction chemotherapy with either FOLFOX6 or carboplatin/paclitaxel and were subsequently evaluated with PET scan.PET non-responders switched to the opposite arm during chemoradiation.Median OS was 48.8 mo for PET responders and 27.4 mo for PET non-responders.Among patients who did not respond to induction chemotherapy,18% in the induction FOLFOX arm and 20% in the carboplatin/paclitaxel arm achieved pCR by switching to the alternative regimen during chemoradiation[68,75,76] .Remarkably,pCR rates in responders that received induction with FOLFOX reached 40.3%[76].
E1201 is a phase II randomized clinical trial[77] comparing neoadjuvant chemoradiotherapy regimens of cisplatin-paclitaxel and cisplatin-irinotecan in patients with resectable adenocarcinomas of the esophagus and GEJ.At the end of the study,81 patients had received trimodality treatment and were evaluable for response rate,DFS and OS.The GEJ was the primary tumor location in 60 patients.Patients received preoperative chemotherapy and radiation and postoperative chemotherapy.Median OS reached 35 mo in cisplatin-irinotecan arm and 5-year OS was 46%,whereas in cisplatin-paclitaxel arm median OS was 21 mo and 5-year OS was 27%.Median PFS was 39.8 mo in cisplatin-irinotecan arm and 12.4 mo in cisplatin-paclitaxel (=0.046).Investigators decided that there was no significant advantage with the use of any of these two regimens,in comparison to other chemoradiotherapy combinations from other studies[77].
In the randomized,multi-center phase II RTOG 0114 study[78],78 patients with resected GC were randomized to receive adjuvant chemotherapy with cisplatinpaclitaxel and fluorouracil,or cisplatin-paclitaxel alone.Both arms would receive subsequent chemoradiation with either infusional 5-FU or infusional paclitaxel and 45 Gy of radiation.Rate of grade 3 or higher adverse events was very high in the triplet chemotherapy arm,thus this cohort was terminated early.Patients who received cisplatin-paclitaxel achieved a 2-year DFS of 52%.This study failed to surpass the 67%2-year DFS from INT0116 study,which was the study's primary endpoint[78].
In the Alliance N0849 randomized phase II trial[79],extended neoadjuvant chemoradiotherapy was compared to standard chemoradiotherapy in patients with locally advanced adenocarcinomas of the esophagus and GEJ.Patients in the extended arm received a combination of docetaxel,oxaliplatin and capecitabine,followed by 5-FU and oxaliplatin concurrently with 50.4 Gy radiation,while patients in the control arm received only chemoradiation.The study’s interim analysis included 42 randomized patients.Among them,71% had stage III disease and 55% and 40%adenocarcinoma of the esophagus and GEJ,respectively.The primary endpoint was pCR rates,which did not differ significantly between the two arms (33% with extended neoadjuvant therapy and 48% with chemoradiotherapy alone).Rate of grade 4 adverse events was numerically higher in the experimental arm (38%24%),although this difference did not reach statistical significance[79].
The phase III CALGB 80101 trial[80] compared two different chemotherapy regimens to be used as part of postoperative chemoradiation treatment plan in patients with gastric or gastroesophageal adenocarcinomas.546 patients who underwent curative resection were randomized to receive either combination of 5-FU and leucovorin,or postoperative combination of epirubicin,cisplatin and 5-FU,before and after concurrent radiotherapy and 5-FU.5-year OS was 44% in both arms,with no difference in any subgroups[80].
In the phase II/III PRODIGE5/ACCORD17 trial[81],FOLFOX regimen was compared to combination of cisplatin-5-FU in terms of safety and effectiveness in patients with esophageal carcinoma of various histologies,receiving definitive chemoradiation.134 patients were randomized to FOLFOX group and 133 to cisplatin-5-FU group.In each group,85% of patients had squamous histologic type.Median PFS was 9.7 mo in the FOLFOX arm and 9.4 mo in the cisplatin-5-FU arm.One death attributed to toxicity took place in the FOLFOX arm and 6 in the cisplatin-5-FU.Rates of grade 3/4 toxicities were similar between the two cohorts.In general,paresthesia and sensory neuropathy was significantly more common in the FOLFOX arm,while serum creatinine increase and mucositis were significantly more frequent in the cisplatin-5-FU arm[81].
A single arm pilot study by Wo[82] examined the use of induction FOLFIRINOX before chemoradiation with carboplatin and paclitaxel in patients with locally advanced gastric and gastroesophageal cancer undergoing surgical resection.25 patients were enrolled,and 20 patients underwent surgical excision and were evaluable for response.At an interim analysis,37% of the evaluable patients had achieved pCR.Grade 3+ toxicities occurred in 28% of patients.This regimen will be evaluated in further trials[82].
Several chemotherapy regimens have been used adjunct to radiation therapy in the pre-or postoperative setting of gastric and gastroesophageal cancer.The most frequently used is the regimen of carboplatin and paclitaxel from the CROSS study,or the combination of cisplatin and 5-FU.Recent data from the PRODIGE5/ACCORD17 study suggest a clear role for the use of FOLFOX,since it achieved similar results with cisplatin-5-FU with no added toxicity.Triplet regimen of cisplatin,paclitaxel and 5-FU followed by radiation was deemed too toxic in the RTOG 0114 study.Finally,the CALGB 80803 study used a more creative approach,by incorporating intermittent restage with PET-CT and shifting non-responders from FOLFOX or carboplatin,paclitaxel to the other regimen,achieving pCR rates in patients with initially resistant tumors.
Ongoing clinical trials
Several ongoing clinical trials attempt to answer clinical questions posed from previous studies or explore novel treatment modalities (Table 5).The multi-center,randomized,phase II CRITICS II trial[83] explores the ideal treatment preoperative modality in patients with resectable GC.Enrolled patients will be randomized in 3 arms,to receive either 4 cycles of neoadjuvant chemotherapy with docetaxel,oxaliplatin and capecitabine,or 2 cycles of DOC followed by chemoradiotherapy with a combination of carboplatin and paclitaxel with 45 Gy radiation,or chemoradiation alone.Primary endpoint is event-free survival at 1 year after randomization,which includes local,regional or distant disease progression and death from any cause[83].The phase III multicenter,randomized NEO-CRAG trial (NCT01815853) evaluates the safety and efficacy of adding 45 Gy radiation to perioperative chemotherapy with 6 cycles of capecitabine and oxaliplatin (CAPOX) in patients with resectable,locally advanced GC.Similarly,the phase III randomized PREACT study[84] compares preoperative chemotherapy to chemoradiotherapy in patients with locally advanced gastric or GEJ adenocarcinomas,planned to undergo surgical resection.Chemotherapy consists of 3 preoperative cycles of S-1 and oxaliplatin (SOX),while chemoradiation consists of two cycles of SOX and concurrent radiotherapy.Patients in both arms will receive postoperative chemotherapy with 3 cycles of SOX.Primary endpoint is an increase in 3-year DFS with chemoradiotherapy regimen.The study also aims to identify microRNAs as a potential predictive biomarker of response to chemoradiotherapy[84].
The King didn t pause to ponder long, for what, thought he, could be in my palace without my knowing about it--the thing is absurd; so he answered quickly: Yes, I promise that you shall have it
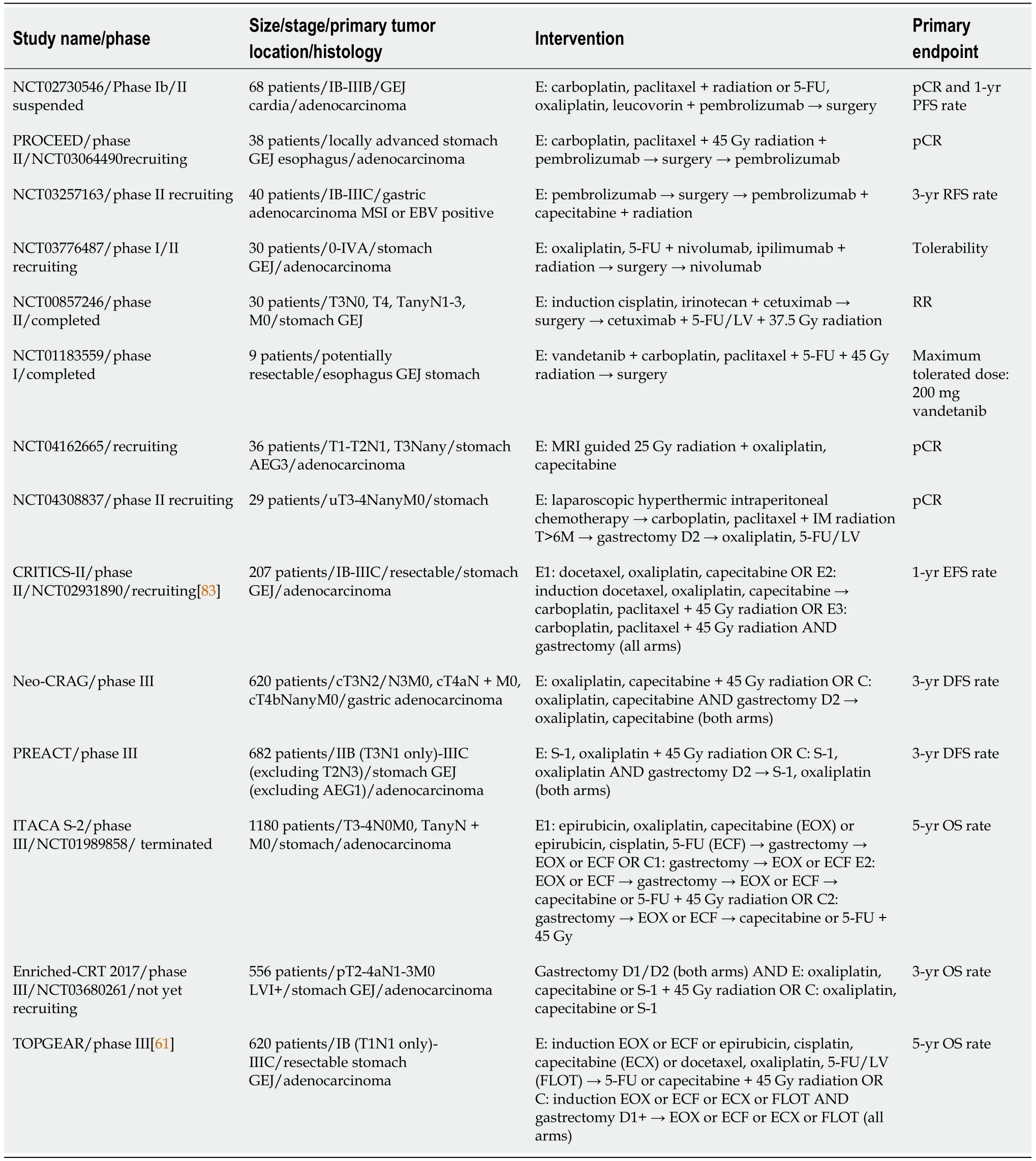
A study that attempts to answer different questions with a complex design is ITACA-S2 (NCT01989858).ITACA-S2 is a randomized,multi-center,phase III trial in which patients with operable GC are randomized into 4 arms of different perioperative treatment modalities.Patients in arm A will receive perioperative chemotherapy with three cycles of combination of epirubicin,cisplatin and capecitabine or 5-FU,and will continue the same regimen after surgery.In arm B,patients will receive the same regimen only after gastrectomy.Arms C and D include the same chemotherapy regimens,with the addition of adjuvant chemoradiotherapy with 45 Gy radiation concurrently with 5-FU or capecitabine.The aim of this study is to evaluate the effect of preoperativepostoperative chemotherapy on OS,and also to assess the benefit of postoperative chemoradiotherapy.Finally,the ENRICHED phase III multicenter randomized clinical trial (NCT03680261) compares adjuvant chemotherapy with 6 cycles CAPOX or SOX to adjuvant chemoradiotherapy with 45 Gy radiation concurrently with capecitabine or S-1,followed by 3 cycles of CAPOX or SOX,in patients with resected lymph node positive GC.Primary endpoint of the study is 3-year OS.
Several trials are currently exploring the role of adding concurrent anti-PD1 checkpoint inhibitor pembrolizumab to neoadjuvant chemoradiotherapy in patients with resectable gastric,GEJ and esophageal carcinoma of different histologic types[85](NCT02730546,NCT03064490,NCT03257163).Furthermore,in a small pilot phase I/II study,addition of combination immunotherapy of nivolumab and ipilimumab to chemoradiation after induction chemotherapy,is being explored in patients with resectable GC (NCT03776487).Newer studies are also focusing on a combination of trimodality treatment and targeting agents,such as the anti-EGFR antibody cetuximab and the VEGFR,EGFR and RET inhibitor vandetanib in the treatment of early GC(NCT00857246,NCT01183559).
Optimization of the radiation component of trimodality treatment is an important part of ongoing trials.NCT04162665 is evaluating magnetic resonance imaging-guided radiotherapy and NCT04523818 the safety and effectiveness of a shorter radiation course in patients with resectable adenocarcinoma (NCT04523818).In the Danish CURE study[86],ctDNA is being used as a predictive biomarker to response and disease progression after different treatment modalities,including chemoradiation in patients with gastroesophageal cancer (NCT04576858).A phase II trial still in recruitment is exploring the role of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy during diagnostic laparoscopy,followed by neoadjuvant chemoradiation,for locally advanced GC (NCT04308837).
Combination of different treatment modalities is an ongoing research field in early gastric cancer,due to sub-optimal results of the current standard of care.Many phase III studies such as TOPGEAR,CRITICS II,NEO-CRAG and PREACT will offer
“Stop a bit, we are just coming to him. It was on the third day, there came marching cheerfully along to the palace a little personage, without horses or carriage, his eyes sparkling like yours; he had beautiful long hair, but his clothes were very poor.”
The father is not terribly concerned about the future of his daughter. He is worried about his impoverishment53, and he does not hesitate to chop off her hands. He is a frustrated54 man, concerned about his inability to succeed- perhaps his virility-and he finds a way to vent43 his frustration55 by attacking his child and then rationalizing it. He simply expects her to forgive because he cannot help himself, because he is afraid of the devil. His violation56 of her is not treated as a crime but rather as an emergency; she is made to feel guilty if she does not relent. (Brothers, 172-173).
concrete data on whether addition of radiation therapy to the newest preoperative chemotherapy regimens,such as FLOT,impacts clinical outcomes.ITACA-S2 is a multi-arm comprehensive study evaluating both the ideal regimen and timing of perioperative treatment,while the ENRICHED trial repeats the adjuvant chemoradiation design in a modern setting.Finally,a significant number of trials incorporated different checkpoint inhibitors in the preoperative setting,after the success of Checkmate 577.
CONCLUSION
Complete surgical resection of the tumor provides the best chance for cure;however,a significant proportion of patients presents with unresectable disease.Adjunctive therapy besides gastrectomy is recommended in a multidisciplinary approach and preoperative therapy is the cornerstone of management in the West.The evidencebased approach should include perioperative chemotherapy or postoperative chemoradiotherapy for selected patients.Similarly,for resectable GEJ cancers,preoperative chemoradiotherapy will enhance surgical outcomes and improve the pCR rate.Evidence from the ARTIST 2 and CRITICS study do not support the adjuvant use of radiation therapy in adequately resected tumors.In the preoperative setting,although prospective data comparing neoadjuvant chemotherapy to chemoradiotherapy are still immature,there is a trend to improved local control and pCR rates,which may translate into significant clinical outcomes in the future.Long term results from the Neo-AEGIS and TOPGEAR trial are eagerly awaited.Moreover,future research should also focus is on optimizing the chemotherapy regimen,defining the role of radiotherapy and immunotherapy and exploring the effect of treatment timing(preoperative,postoperative or both).
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
