Cognitive impairment and its association with circulating biomarkers in patients with acute decompensated heart failure
Ying-Chang Tung, Fu-Chih Hsiao, Chia-Pin Lin, Wen-Chuin Hsu, Pao-Hsien Chu,3,4,?
1. Division of Cardiology, Department of Internal Medicine, Taipei, Taiwan, China; 2. Department of Neurology, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taipei, Taiwan, China; 3. Dementia Center,Chang Gung Memorial Hospital, Taoyuan, Taiwan, China; 4. Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taipei, Taiwan, China
ABSTRACT
With increased longevity and improved survival from coronary artery disease,the prevalence and health care burden of heart failure (HF) have been growing considerably over time. Cognitive impairment (CI) is a common geriatric condition in patients with HF,with an incidence of 25%–85%.[1,2]In a cross-sectional survey of subjects aged 65 years or older, HF conferred an approximately 2-fold increased risk of CI.[3]CI in patients with HF may impair their somatic awareness, self-management, and adherence to guideline-recommended therapies, leading to increased risks of mortality and hospitalization.[4]Compared with ambulatory patients with HF, patients who are hospitalized for acute decompensated HF (ADHF) may be more frequently underrecognized for the coexisting CI[5]due to the worsening symptoms and concomitant acute illnesses that may demand more medical attention than CI. Given its adverse impact on medication compliance and longterm outcomes, CI should be incorporated into the multidisciplinary care of these patients.
The pathophysiologic link between HF and CI remains unclear. The concept of heart–brain connection proposes that cardiovascular risk factors, such as diabetes, hypertension, dyslipidemia, atrial fibrillation, and smoking, can lead to the development of cerebrovascular disease and its sequelae, including CI and dementia.[6,7]Patients with HF often have at least one of these risk factors and thus tend to develop concomitant CI. HFper semay also lead to cognitive decline through systemic and cerebral hypoperfusion.[8]In addition, raised inflammatory mediators in HF, such as interleukin-1, interleukin-6,and tumor necrosis factor-α, impair cognitive processes by modulating neurogenesis, synaptic plasticity, and neurotransmitter cascades involved in learning and memory.[9]The associations between clinical parameters and cognitive function in patients with HF have been extensively analyzed. Reduced left ventricular ejection fraction (LVEF) or cardiac output,[10]advanced New York Heart Association (NYHA) functional class,[11]and elevated Btype natriuretic peptide (BNP) levels[12]reflect an increased severity of CI. However, novel HF biomarkers, such as growth/differentiation factor-15 (GDF-15), galectin-3, and clusterin, or biomarkers that are mechanistically linked to CI or dementia, such as tau[13]and amyloid-beta 1–40 (Aβ40) and 1–42(Aβ42),[14]have rarely been evaluated for their association with CI in the HF population.
This prospective observational study investigated the incidence of CI in patients who were admitted for ADHF and observed their cognitive changes over the treatment course of HF. Given the interconnected pathophysiology between HF and CI, we hypothesized that incorporating biomarkers related to HF or cognitive dysfunction may improve the risk stratification of CI and clinical outcomes beyond the traditional risk predicting models for patients with HF.
METHODS
Study Design and Patient Enrollment
This prospective, observational cohort study was conducted at the Heart Failure Center, Linkou Chang Gung Memorial Hospital, Taiwan. Patients who were admitted for ADHF with LVEF < 40% and survived to hospital discharge were consecutively enrolled from December 2016 to August 2019. The majority of patients were recruited from the emergency department, and some through referrals from the outpatient clinics or other departments of our hospital. The patients were screened by reviewing electronic medical records for admission diagnoses within 48 h of hospitalization. The patients were interviewed and enrolled before discharge once they had been stabilized after initial management. The exclusion criteria were the following: (1) being < 20 years of age, (2) having clinically overt dementia,delirium, major depression, or other psychiatric diseases, (3) having terminal cancer or life expectancy< 1 year, and (4) unwilling to provide informed consent. The patients either started in-hospital rehabilitation or were instructed to perform stepwise physical exercise after discharge, based on the assessment of their functional capacity by a cardiac rehabilitation specialist. All patients underwent routine laboratory tests, echocardiography, and biomarker assays and were assessed for cognitive function, depressive symptoms, and health-related quality of life during the index hospitalization and at three months and one year after discharge. For those who were readmitted for HF in the study period, additional examinations were conducted during each rehospitalization.
All participants provided written informed consent. The study protocol complied with the ethical standards outlined in the 2013 version of the Helsinki Declaration and was approved by the Institutional Review Board of Chang Gung Memorial Hospital (No. 201600737B0).
Assessment of Cognitive Function, Depressive Symptoms, and HF-Related Quality of Life
Cognitive function was evaluated using the Mini-Mental State Examination (MMSE; PAR, Florida,USA) and the Mini-Cog test. The MMSE consists of 11 questions that test orientation to time and place,short-term memory, attention and calculation, and visual spatial construction using a 30-point scale. The Mini-Cog test is a 3-min instrument used to screen cognitive dysfunction consisting of a three-item recall test for memory and a simply scored clockdrawing test. CI was defined as MMSE score ≤ 26[15]or Mini-Cog score ≤ 2[16]at enrollment during hospitalization (baseline). Follow-up assessments were performed at 3 months and 1 year after discharge to observe cognitive changes. For those considered to have CI, a neurologist was consulted to confirm the diagnosis and perform a thorough neurologic examination. Although major depression was an exclusion criterion in this study, considering the coexistence of depressive symptoms in patients with HF or CI, we evaluated depressive symptoms with the Beck Depression Inventory-II (BDI-II), which is a 21-item, self-report measure of depressive symptoms,with a total score of 0–63.[17]A higher total BDI-II score indicates more severe depressive symptoms.The Kansas City Cardiomyopathy Questionnaire(KCCQ) was used to quantify the patients’ symptoms, physical and social function, and quality of life,[18]with higher KCCQ scores indicating less severe HF symptoms or functional limitations and a better quality of life. The BDI-II and KCCQ were administered simultaneously with cognitive assessment.
Biomarker Assays
Fasting blood samples were drawn from the antecubital vein of the participants on the early morning of cognitive assessment during hospitalization and were then centrifuged at 1 500 g for 10 min and stored at ?80°C until thawed for biomarker analysis. BNP (Triage BNP Test; Biosite Diagnostics, San Diego, CA, USA) was analyzed along with other routine laboratory tests, including complete blood cell counts, liver and kidney function,fasting glucose, HbA1C, and electrolytes. Other cardiac biomarkers analyzed were GDF-15, galectin-3,and clusterin (Human Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA). The detection range was 93.6–7 500 pg/mL for GDF-15, 1.252–40 ng/mL for galectin-3, and 6.26–400 μg/mL for clusterin. These biomarkers were selected on the basis of their biological association with both HF and CI.[19–23]We also analyzed circulating levels of total tau (t-tau) and amyloid-beta 1–40 (Aβ40) and 1–42(Aβ42) peptides (Human ELISA Kit, Invitrogen,Carlsbad, CA, USA), given their pathophysiologic roles in Alzheimer’s disease and their association with cardiovascular risk factors and outcomes.[24]The detection range was 62–4 000 pg/mL for tau,9.8–500 pg/mL for Aβ40, and 15.6–1 000 pg/mL for Aβ42.
Covariates
Demographic characteristics included age, sex,years of education, and economic status. Clinical covariates included prior history and etiology of HF,NYHA functional class, hemodynamic data, comorbidities, smoking, alcohol, LVEF, and medication use. NYHA functional class was determined by the primary care physicians based on the patient’s symptoms on the day of enrollment during index hospitalization and at the 3-month and 1-year follow-up.
Follow-up and Clinical Outcomes
All patients were followed up monthly in the first three months after discharge and then every 3 months up to 1 year, with additional visits, as required, in case of clinical exacerbation. The primary endpoint,a composite of all-cause mortality or hospitalization for HF at 1 year, was compared between patients with and without CI. Clinical events were ascertained at follow-up visits and through medical record review.
Statistical Analysis
The patients’ clinicodemographic characteristics between the CI and non-CI groups were compared using an independent samplesttest for continuous variables and Fisher’s exact test for categorical variables. The data of BNP between groups were compared using the Mann–WhitneyUtest because of its nonnormal distribution. The changes in the MMSE,Mini-Cog, BDI-II, and KCCQ scores from baseline to 3-month and 1-year follow-up were analyzed using the generalized estimating equation (GEE),where time was the only explanatory factor. The changes in the scores between groups (CIvs. non-CI) were compared using GEE, which included the main effects of group, time (treated as a categorical variable), and the interaction effect of group by time.The changes between groups were considered different if the interaction effect was significant. In the GEE model, the working correlation matrix was exchangeable, the link function was identity, and the distribution was normal. The receiver-operating characteristic (ROC) analysis was used to evaluate the capacity of the biomarkers to discriminate CI at index hospitalization. The optimal cutoff was determined using the Youden index. The linear trend for temporal changes of MMSE was tested using GEE stratified by the optimal cutoff of biomarkers(i.e., GDF-15). The relationship between GDF-15 levels and MMSE scores was examined using Spearman’s rank correlation. The Cox proportional hazards model was used to analyze the association between the biomarkers and the primary outcome of all-cause mortality or hospitalization for HF. In an alternative Cox model, the patients were stratified into four groups according to the GDF-15 cutoff and the presence of CI. To avoid overfitting, we incorporated only age, LVEF, and NYHA functional class (IVvs. II–III) in the multivariable Cox model.A two-sidedP< 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25 (IBM SPSS, Chicago, IL,USA).
RESULTS
Patient Characteristics
We enrolled 145 patients (Table 1). The mean age of the study cohort was 61.8 ± 13.4 years, and 80.6%of them were men. The average years of education were 9.6 years (SD = 4.2 years). Furthermore, 64.7%of the patients had new-onset HF, with ischemic heart disease being the major etiology (46.8%). Most patients presented as NYHA functional class III(49.6%) or IV (44.6%), with mean LVEF of 29.9% ±11.2% and median BNP of 1102 pg/mL (interquartile range 506–1970 pg/mL). Fifty-four (37.2%) patients had CI at enrollment. Compared with patients without CI, those with CI were older, had lower education levels, had a higher prevalence of prior HF and concomitant CKD, had a lower level of hemoglobin, and received beta blockers more frequently for HF treatment. No significant between-group difference was observed in other covariates.
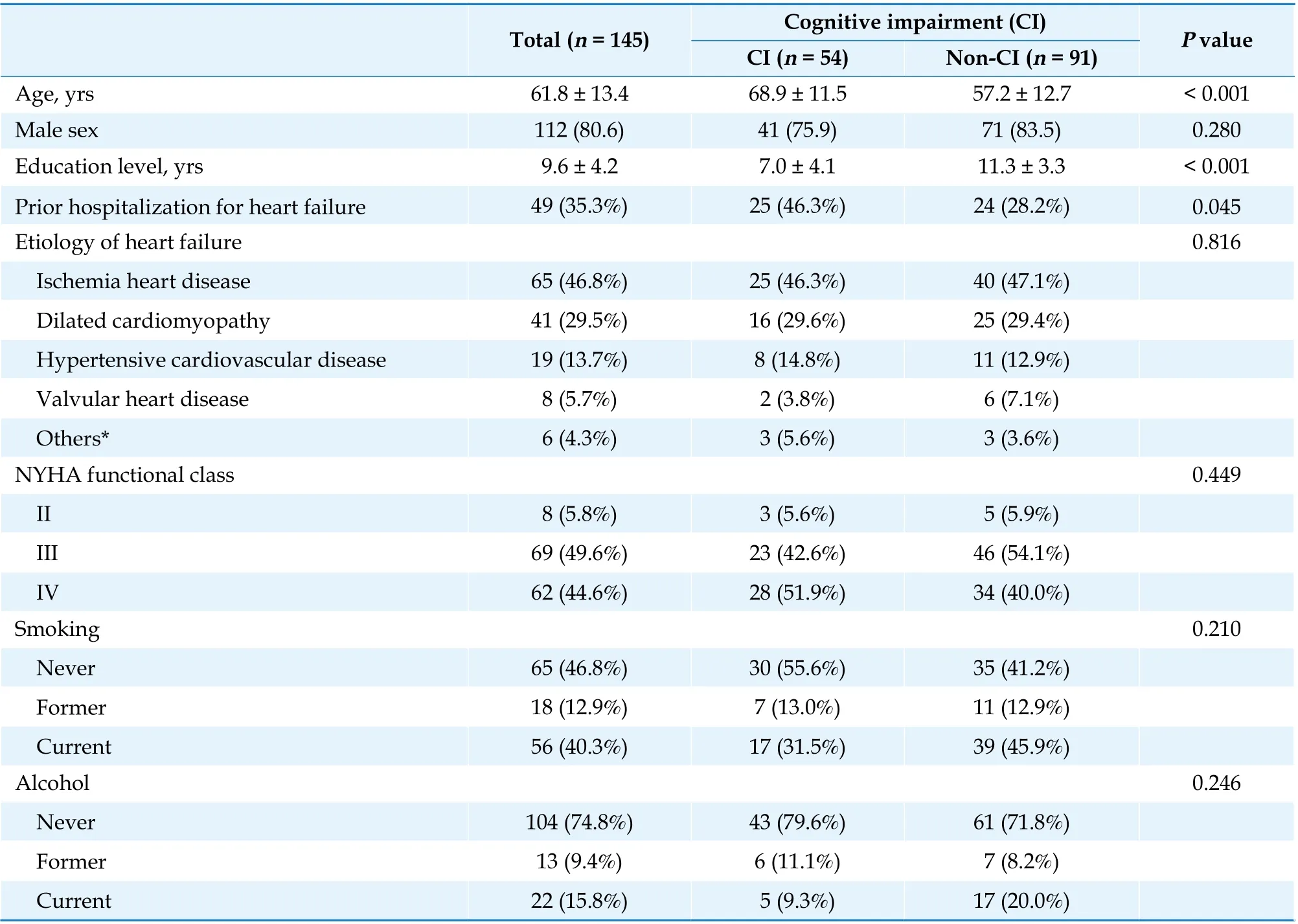
Table 1 Baseline characteristics of study patients according to their status of cognitive impairment.
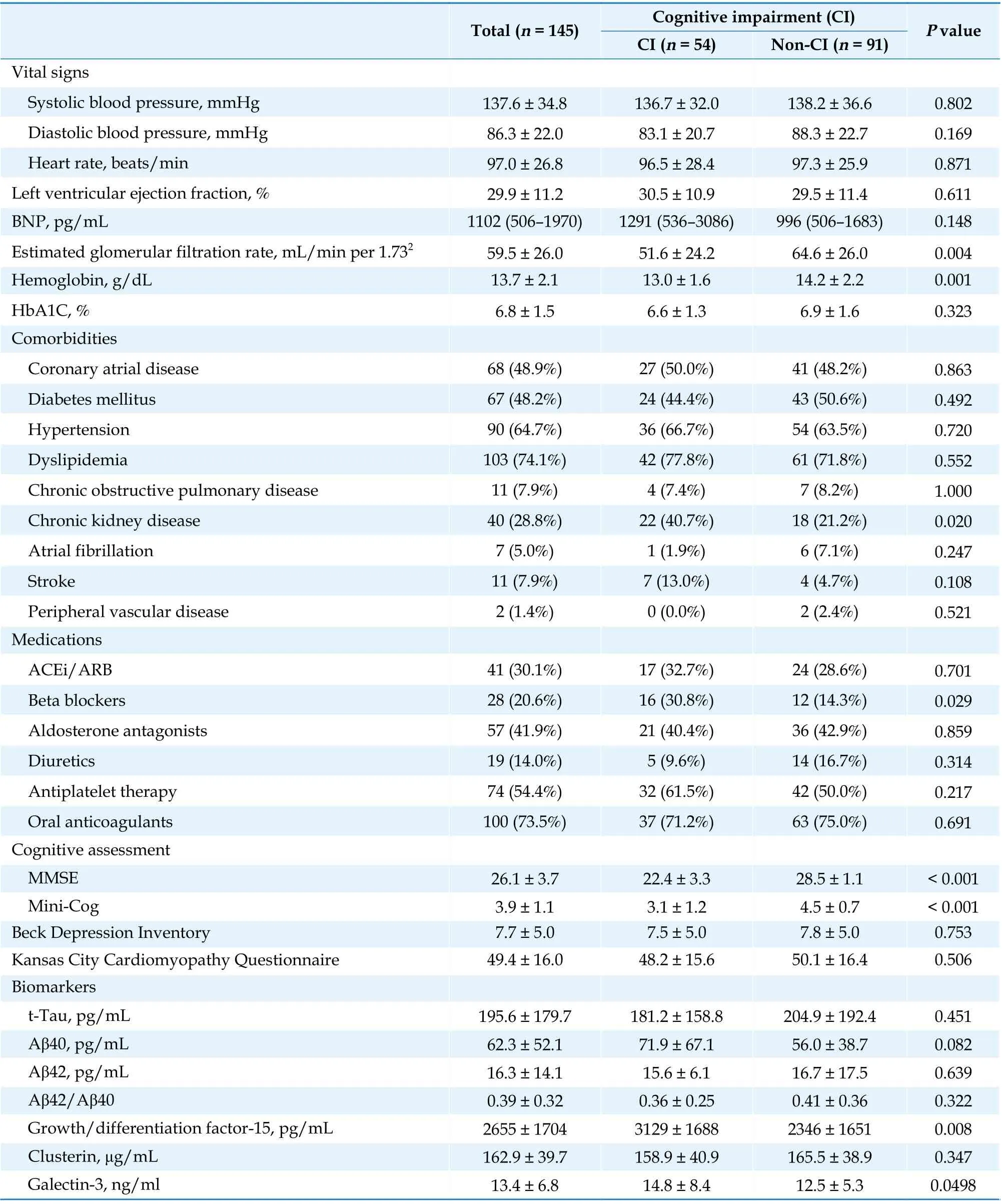
Continued
Cognitive Assessments and Changes in Cognition Over Time
At baseline, the CI group had significantly lower MMSE (22.4vs. 28.5;P< 0.001) and Mini-Cog scores(3.1vs. 4.5;P< 0.001) compared with the non-CI group (Table 1). In the whole cohort, the mean MMSE scores at 3-month and at 1-year follow-up were both significantly higher than that at baseline. Notably, the improvement in the MMSE scores was mainly driven by the CI group (Figure 1A). However,the Mini-Cog scores did not significantly change over time in the overall study cohort or in the two subgroups (Figure 1B).
Depressive Symptoms and HF-specific Quality of Life
Both CI and non-CI groups had only minimal depressive symptoms at baseline, as measured using the BDI-II (7.5 and 7.8;P= 0.753). The BDI-II score significantly decreased at 3 months and 1 year for the whole cohort (bothP< 0.001), and this improvement was similar between the CI and non-CI groups(Figure 1C). The KCCQ scores were also comparable between the two groups during hospitalization (48.2vs. 50.1;P= 0.506). The KCCQ scores increased substantially at three months and 1 year for the whole cohort (bothP< 0.001), but the improvement was not different between the two groups (Figure 1D).
Correlation between Biomarkers and CI at Enrollment
The patients with CI had significantly higher levels of GDF-15 (3 129vs. 2 346 pg/mL;P= 0.008)and galectin-3 (14.8vs. 12.5 ng/mL;P= 0.049 8)than those without CI (Table 1). No significant difference was observed between the two groups in BNP, clusterin, t-tau, or Aβ42 levels or the Aβ42/Aβ40 ratio, although there was a trend toward higher Aβ40 in patients with CI (71.9vs. 56.0 pg/mL;P= 0.082). Table 2 presents the results of the ROC analysis for the performance of biomarkers to discriminate CI at enrollment. The results revealed that only GDF-15 levels could discriminate CI with a modest performance (area under the curve = 63.9%,95% confidence interval: 54.8%–73%). The optimal cutoff was 1 621.1 pg/mL, with a sensitivity of 85.2% (95% confidence interval: 72.9%–93.4%) and a specificity of 40.5% (95% confidence interval:30.2%–51.4%). When the GDF-15 levels were stratified by the cutoff, the patients with higher GDF-15 levels had a significant increase in MMSE scores,whereas those with lower GDF-15 levels did not(Figure 2). Scatter plots indicated a negative correlation between GDF-15 levels and baseline MMSE scores (Spearman’s rank correlation [ρ] = ?0.32,P<0.001; Figure 3A) and a positive correlation between GDF-15 levels and extent of improvement in MMSE scores at 3 months (ρ = 0.24,P= 0.009; Figure 3B)and at 1 year (ρ = 0.20;P= 0.048; Figure 3C).
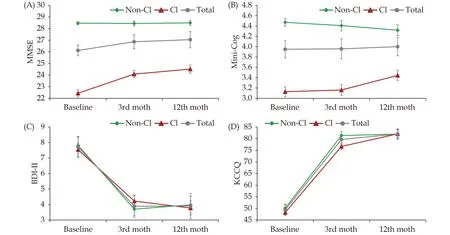
Figure 1 Temporal changes in cognitive function assessed by the MMSE (A) and the Mini-Cog (B) and in the depressive symptoms assessed by the BDI-II (C) and heart failure-specific quality of life by the KCCQ (D). ‘*’: Significant difference versus baseline for the whole cohort; ‘#’: significant difference of the change values from baseline to follow up between the CI and non-CI groups. BDI-II:Beck Depression Inventory-II; CI: cognitive impairment; MMSE: Mini-Mental State Examination; KCCQ: Kansas City Cardiomyopathy Questionnaire.
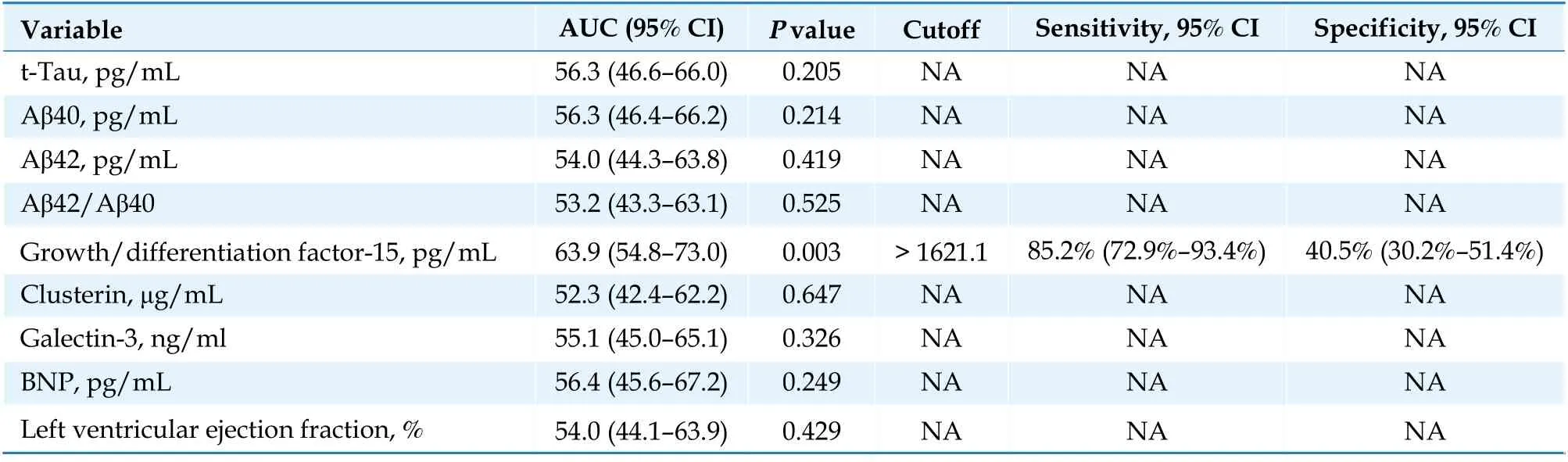
Table 2 Receiver-operating curve analysis of biomarkers for the discrimination of cognitive impairment.
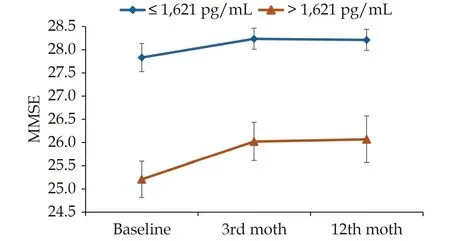
Figure 2 Temporal changes in cognitive function assessed by the MMSE stratified by the optimal cutoff of GDF-15. GDF-15:growth/differentiation factor-15; MMSE: Mini-Mental State Examination.
Association between Biomarkers, CI, and HF Outcomes
The primary endpoint occurred in 30 patients(20.7%) at 1 year, including 9 deaths (6.2%) and 26 hospitalizations for HF (17.9%; Table 3). After adjustment for age, LVEF, and NYHA class (IVvs.II–III), a greater level of GDF-15 was significantly associated with an increased risk of the primary endpoint (hazard ratio [HR] = 1.44; 95% CI 1.19–1.75),whereas a higher level of clusterin was associated with a reduced risk of the primary endpoint (HR 0.30; 95% confidence interval: 0.11–0.80). We further stratified the study patients into four groups according to the GDF-15 cutoff of 1621.1 pg/mL and the presence of CI (Supplement Table). In the univariate analysis, the patients with CI with or without elevated GDF-15 levels had a higher risk of the primary endpoint than patients without CI(GDF-15 > 1621.1 pg/mL: HR = 3.7; 95% confidence interval: 1.04–13.10; GDF-15 < 1621.1 pg/mL: HR 5.79, 95% confidence interval: 1.17–28.73). Among the patients without CI, those with elevated GDF-15 levels had a nonsignificantly higher risk of primary endpoint than those with low GDF-15 levels (HR 2.93; 95% confidence interval: 0.83–10.37;P= 0.096).However, the potential effect of CI and GDF-15 on the outcomes was attenuated in the multivariate analysis (Supplemental Table).
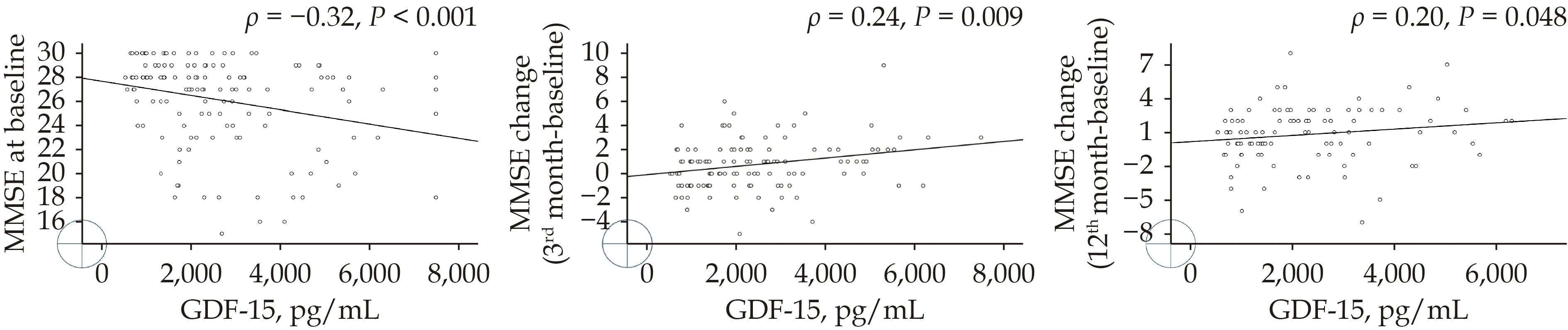
Figure 3 The relationship between levels of GDF-15 and the baseline MMSE (A), the changes in MMSE at 3 months (B), and the changes in MMSE at 1 year (C). GDF-15: growth/differentiation factor-15; MMSE: Mini-Mental State Examination.
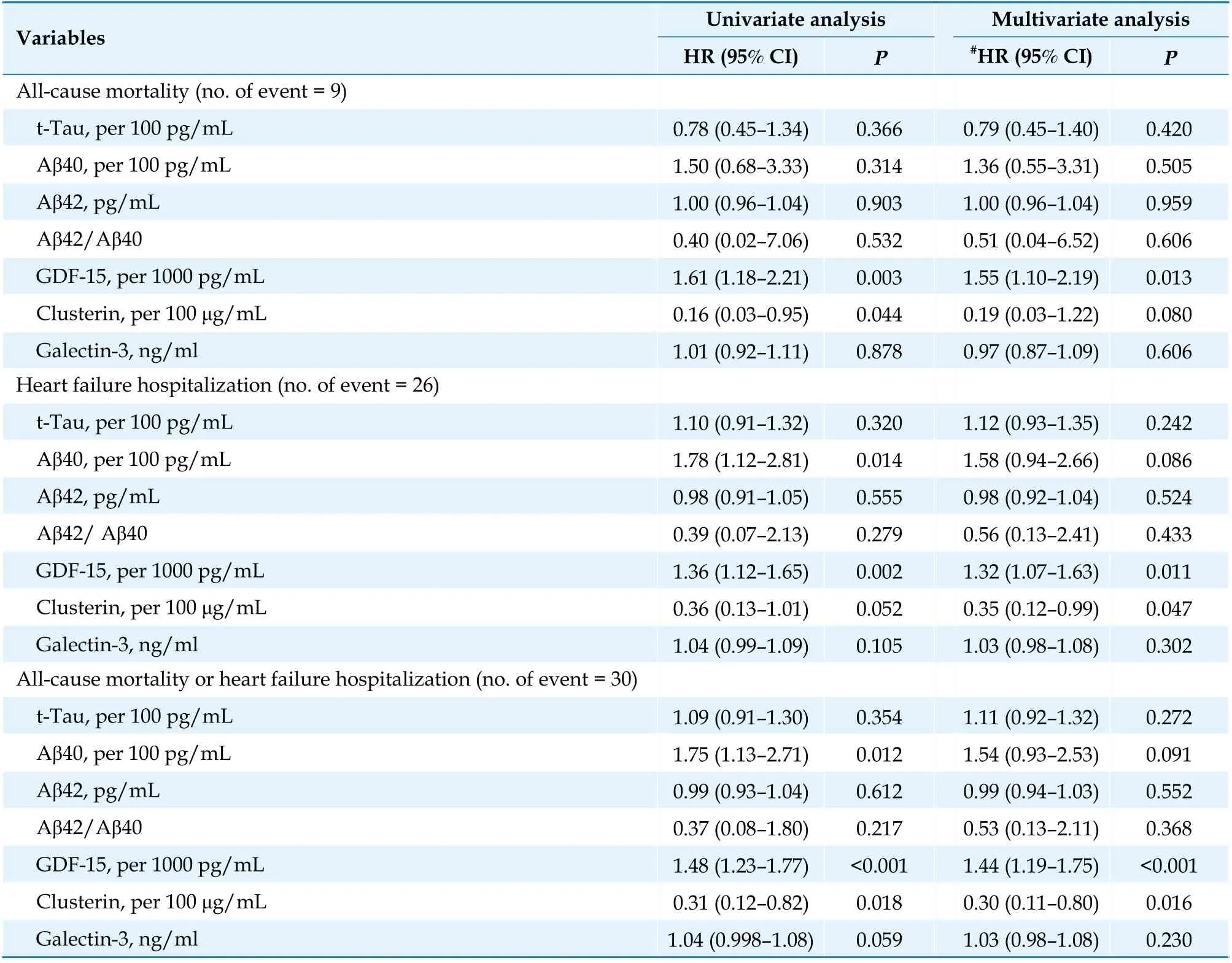
Table 3 Association of biomarkers and clinical outcomes at one year.
DISCUSSION
This prospective observational cohort study evaluated the incidence of CI, changes in the cognitive function at follow-up, and association between biomarkers and CI in patients who were admitted for ADHF and had LVEF < 40%. The major findings are as follows: (1) the incidence of CI was 37.2% in our cohort; (2) the patients with CI had gradual improvement in cognitive function, as measured using the MMSE, but did not reach the level of those without CI, in whom there was no significant cognitive change over time; (3) the improvement in depressive symptoms and HF-specific quality of life was comparable between the patients with and without CI; (4) among the biomarkers related to HF or cognitive dysfunction, only GDF-15 predicted the presence of CI with moderate discrimination capacity and was associated with worse HF outcomes.
Incidence of CI and Temporal Changes in Cognitive Function
The rate of CI in this study was within the wide range of 25%–80%, as indicated by previous studies on HF populations.[1,2]The main reasons for this wide variation include the differences in study designs, patient enrollment criteria, and cognition assessments and cutoffs used to define CI. Our study excluded overt symptoms of dementia, delirium, or major depression, and hence, may have underestimated the rate of CI, given the frequent coexistence of these conditions. The direction of cognitive change varies according to the type of intervention treatment applied, duration of follow-up, and comparator groups.[1]Cognition in hospitalized patients with HF tends to improve compared with their own baseline assessments, particularly when the length of follow-up is less than 1 year, with some reports showing improvement to levels similar to those with stable HF.[25]By contrast, studies with longer follow-ups more frequently report cognitive decline.[26]The overall improvement in cognitive function in this study was derived mainly from the increased MMSE in the patients with CI.However, we could not determine if the cognition of patients with CI had recovered to their real baseline levels before HF worsened. It is conceivable that the gap in the cognitive function between the CI and the patients without CI may have existed even before enrollment, because the patients with CI were older, less educated, and more frequently had a prior history of HF, all of which have been shown to increase the risk of CI in this population.[1,2]However, although the patients without CI had relatively stable cognitive performance at one year, they are still at risk for future decline. Prior longitudinal studies have demonstrated that a high baseline MMSE is independently associated with cognitive decline in patients with HF with reduced LVEF.[4]Therefore, longer follow-up is mandated to determine the longitudinal trajectories of cognitive function in these patients.
Association between Cognitive Function, Depressive Symptoms, and HF-specific Quality of Life
The improvement in the MMSE score, particularly in the CI group, was accompanied by an improvement in the BDI-II and the KCCQ scores. This observation indicates a dynamic and interconnected relationship between CI and depressive and HF symptoms in these patients. The mechanistic correlation between these conditions, although not well understood, may be inferred from their common pathophysiological characteristics, such as neurohormonal activation, inflammatory mediators, vascular damage, and psychosocial and behavioral factors.[9,27]The recovery from HF symptoms and improvement in quality of life, as indicated by the substantially increased KCCQ score, may partly explain a further reduction in the BDI-II score despite the relatively low BDI-II at baseline. Studies on physical exercise or rehabilitation programs have shown beneficial effects on cognitive and depressive symptoms.[28]Although we did not specifically assess physical function in this study, all patients were instructed to engage in stepwise physical exercise based on their functional capacity, which might have contributed to improvement in the measures of cognition and depressive symptoms in the recovery phase.
Association between Biomarkers, CI, and HF Outcomes
Several studies have consistently demonstrated a relationship between elevated natriuretic peptides and the risk of CI or dementia in patients with HF.[29,30]However, the level of BNP was insignificantly higher in the CI group than in the non-CI group in our study. The small number of patients may account for the lack of statistical significance. In addition, blood samples were obtained on the same day of cognition assessment after initial HF management. The difference in the levels of BNP and other biomarkers between the two groups may have been attenuated by then.
Given the shared risk factors in cardiovascular disease, cerebrovascular disease, and dementia, vascular markers, such as GDF-15, clusterin, and galectin-3, have recently gained research interest as potential markers for cognitive decline.[19–23]In this study, only GDF-15 had a moderate discriminative capability for predicting CI. Furthermore, a higher GDF-15 was associated with a lower baseline MMSE but a greater improvement in cognitive function at follow-up. GDF-15, a stress responsive cytokine that belongs to the transforming growth factor-β family,has recently been shown to be associated with cognitive decline or dementia in the elderly[31]and in the Framingham Offspring cohort,[19]possibly through the mechanisms of inflammation and vascular brain injury.[19,23,31]We also observed an association between GDF-15 and HF outcomes, consistent with its prognostic utility in prior studies. Our findings, although they should be validated in larger studies, may help expand the implication of GDF-15 as a potential marker of CI in patients with HF.
Both CI and elevated GDF-15 have been documented to adversely affect long-term outcomes.However, the prognostic impact of GDF-15 in this study was attenuated when the patients were further stratified by the presence of CI, probably due to the small patient number, relatively low event rates, and short follow-up. Further research with a large sample size and sufficient power is required to investigate the incremental value of biomarkers in combination with traditional HF markers in the risk stratification of CI and clinical outcomes in the HF population.
LIMITATIONS
This study has several limitations. First, this was a single-center study with a small sample size and a relatively brief follow-up for the detection of longitudinal cognitive changes. As mentioned above, we were not able to detect the difference in clinical outcomes in patients with vs. without CI. To avoid overfitting, some parameters were not incorporated in the statistical models to determine the independent predictors of baseline CI, subsequent cognitive changes, or 1-year outcomes. Second, the study design did not include a comparator group (the patients themselves before hospitalization or patients with chronically stable HF) to allow for a comparison of cognitive function before and after HF worsening and to determine the extent of recovery at followup. Third, there has been no consensus on the timing of cognitive assessment in patients with ADHF.The study patients were enrolled and evaluated during hospitalization; our results may have been confounded by residual HF symptoms or symptoms related to other concomitant acute illnesses.The first follow-up cognitive assessment was performed at postdischarge 3 months. An earlier assessment may have seen earlier improvement.Forth, instead of looking into specific cognitive domains, we focused on global cognitive function and temporal cognitive change with the use of the MMSE and the Mini-Cog test. Although the MMSE is widely used in clinical research, it may be limited for its lower sensitivity for detecting CI in patients with HF compared with other instruments.[27]Finally, the lack of brain imaging studies and physical functional assessment hindered us from investigating the pathophysiologic link between HF and structural changes in the brain as well as the impact of physical exercise on the cognitive and depressive measures in our patients.
CONCLUSIONS
In this prospective observational study in patients who were admitted for ADHF, the incidence of CI was 37.2%. The cognitive function in the patients with CI improved gradually at 3 months and 1 year but did not reach the level of those without CI. The recovery of cognition was accompanied by reducing depressive symptoms and improving quality of life. Among the biomarkers that are related to HF or cognitive dysfunction, only GDF-15 predicted the presence of CI with moderate discrimination capacity and was associated with worse clinical outcomes.
FUNDS
This work was supported by research grants from Linkou Chang Gung Memorial Hospital, Taiwan,China (CMRPG3F1633).
ACKNOWLEDGMENTS
The authors would like to thank Alfred Hsing-Fen Lin, MS, Raising Statistics Consultant, for his statistical assistance. Mr. Lin declared no competing interests between the findings of this study and his company.
 Journal of Geriatric Cardiology2022年3期
Journal of Geriatric Cardiology2022年3期
- Journal of Geriatric Cardiology的其它文章
- COVID-19: cardiovascular manifestations—a review of the cardiac effects
- Associations of body mass index and hospital-acquired disability with post-discharge mortality in older patients with acute heart failure
- Mortality in patients with heart failure and suicidal ideation discharged to skilled nursing facilities
- Association of time-varying changes in physical activity with cardiac death and all-cause mortality after ICD or CRT-D implantation
- Severe aortic stenosis and acute coronary syndrome in an elderly patient with idiopathic thrombocytopenic purpura:a therapeutic challenge
- Caseous calcification of mitral annulus in the setting of multivessel disease
