The short form of the SUR1 and its functional implications in the damaged brain
Iván Alquisiras-Burgos, Javier Franco-Pérez, Moisés Rubio-Osornio,Penélope Aguilera,*
Abstract Sulfonylurea receptor (SUR) belongs to the adenosine 5′-triphosphate (ATP)-binding cassette (ABC) transporter family; however, SUR is associated with ion channels and acts as a regulatory subunit determining the opening or closing of the pore.Abcc8 and Abcc9 genes code for the proteins SUR1 and SUR2, respectively.The SUR1 transcript encodes a protein of 1582 amino acids with a mass around 140–177 kDa expressed in the pancreas,brain, heart, and other tissues.It is well known that SUR1 assembles with Kir6.2 and TRPM4 to establish KATP channels and non-selective cation channels, respectively.Abbc8 and 9 are alternatively spliced, and the resulting transcripts encode different isoforms of SUR1 and SUR2, which have been detected by different experimental strategies.Interestingly, the use of binding assays to sulfonylureas and Western blotting has allowed the detection of shorter forms of SUR (~65 kDa).Identity of the SUR1 variants has not been clarified, and some authors have suggested that the shorter forms are unspecific.However, immunoprecipitation assays have shown that SUR2 short forms are part of a functional channel even coexisting with the typical forms of the receptor in the heart.This evidence confirms that the structure of the short forms of the SURs is fully functional and does not lose the ability to interact with the channels.Since structural changes in short forms of SUR modify its affinity to ATP, regulation of its expression might represent an advantage in pathologies where ATP concentrations decrease and a therapeutic target to induce neuroprotection.Remarkably, the expression of SUR1 variants might be induced by conditions associated to the decrease of energetic substrates in the brain (e.g.during stroke and epilepsy).In this review, we want to contribute to the knowledge of SUR1 complexity by analyzing evidence that shows the existence of short SUR1 variants and its possible implications in brain function.
Key Words: brain edema; epilepsy; Parkinson’s disease; stroke; sulfonylurea receptor 1;SUR1; traumatic brain injury; TRPM4
Introduction
The adenosine 5′-triphosphate (ATP)-binding cassette (ABC)transporters is a superfamily of membrane proteins that involve ATPase activity and the subsequent ATP hydrolysis to transport a diversity of substrates across the cell membrane.These proteins are evolutionarily conserved and widely distributed in different phyla.In the human genome, there are 48 genes coding for proteins of the ABC superfamily.The ABC transporters have been classified according to their function as exporters, importers, and non-transporters.In mammals, sequence homology and gene structure have also been used to group them into seven subfamilies (ABCA to ABCG) (Thomas et al., 2020).Under physiological conditions,most ABC transporters function either importing or exporting substances; however, there are exceptions, such as the sulfonylurea receptor (SUR), which is associated with ion channels and acts as a regulatory subunit determining the opening or closing of the channel (Tinker et al., 2018).
SUR is a paradoxical ABC transporter due to its function as a non-transporter.It has been widely described that SUR assembles with Kir6.2, an inward rectifier K+channel, to establish KATPchannels (Puljung, 2018).KATPchannels are expressed in many cell types and tissues, including the brain, ovary, heart, kidney, skeletal, and smooth muscles,where synchronize cell metabolism with electrical activity regulating transmembrane potassium fluxes (Bal et al., 2018;Filipets et al., 2019; Kaya et al., 2019; Kim et al., 2020).Early stoichiometry studies indicated that a 1:1 ratio between Kir6.2 and SUR subunits is necessary for channel assembly.Therefore, the active KATPchannel complex is a hetero-octamer of four Kir6.2 subunits arranged at the center of the complex,forming the channel pore and surrounded by four regulatory SUR subunits (Li et al., 2017) (Figure 1A).Intracellular nucleotides regulate the KATPchannels, thus increasing ADP concentrations open the channel eliciting K+efflux, membrane hyperpolarization, and inhibition of electrical activity.Conversely, ATP closes the channel by coupling in one of the four binding sites located in the Kir6.2 subunits.Although the mechanism is not yet fully elucidated, it has been proposed that SUR determines the KATP channel opening by Mg-ADP binding where Mg2+is necessary to trigger a conformation change in SUR1 (Puljung et al., 2019) (Figures 1Band2).Оn the other hand, sulfonylureas are compounds that inhibit the activity of the KATPchannel expressed in pancreatic β cells, and according to their ability to stimulate insulin secretion, they have been used in the management of diabetes mellitus type 2.Second-generation drugs called glyburide or glibenclamide are 100 to 1000 more potent stimulators of insulin secretion.Regarding the specific mechanism of action, it has been suggested that sulfonylurea inhibition of KATPchannels provokes membrane depolarization, activation of voltage-dependent Ca2+channels, Ca2+influx, and finally Ca2+-dependent insulin granule exocytosis (de Wet and Proks, 2015).
Interestingly, reports have described that SUR is associated with and regulates non-selective channels permeable to monovalent cations (e.g., Na+, K+) such as the transient receptor potential melastatin-4 (TRPM4) (Woo et al., 2020).Similar to KATPchannels, TRPM4 is a hetero-multimeric composed of the co-assembly of four pore-forming subunits and four SUR regulatory subunits (Figure 1A).Electrophysiological analysis has revealed that SUR-TRPM4 is a Ca2+-activated cation channel; therefore, intracellular Ca2+opens the channel and consequently elicits Na+influx.Also,it is reported that binding of intracellular ATP can modulate the TRPM4 activity and probably this modulation could be regulated by SUR.Importantly, TRPM4 dysfunction has been linked to pathological processes (Amarouch et al., 2020)(Figure 2).
There are two SUR isoforms named SUR1 and SUR2, which are encoded by the ABC subfamily C 8 (Abcc8) and 9 (Abcc9)genes, respectively.Two alternative splicing of the last exon of SUR2 gives rise to the SUR2A and SUR2B isoforms.These two peptides differ only in the last 42 amino acids and consequently are considered highly homologous (Yamada and Kurachi, 2005).SUR1 also has splicing isoforms; however, little is known about their identity.Therefore, this review analyzes current information about isoforms and the alternative splice variants for SUR1 to understand the relationship between diversity, complexity, and function.Importantly, SUR1 function is relevant in the central nervous system in some process of neurodegeneration and in some diseases, therefore it can be a target to induce neuroprotection.The purpose of the review was to show the evidence of the possible expression of short forms of SUR1 in the brain.This information might be relevant to understand SUR1 function in the damaged brain and help to resolve problems in forthcoming investigations.
Search Strategy and Selection Criteria
The literature selection in this review was done by exploring terms associated to SUR1 expression, its isoforms, and its function in the brain.An electronic search was performed using the MeSH terms: SUR, SUR1, SUR2, Sulfonylurea Receptors, Abcc8, ATP-binding cassette transporters,glyburide, glibenclamide, TRPM4, K(ATP), ion channel,protein isoforms, splice variant, expression, brain, heart,pancreas, metabolism, ischemic stroke, cerebral hemorrhage,intracerebral hemorrhage, traumatic brain injury, epilepsy,status epilepticus, Parkinson’s, Alzheimer’s, neurodegenerative disease, blood brain barrier, and its combination in the Google scholar and PubMed databases.The results were further screened by title and abstract.Because the first part of the review focused on the description of evidence associated to the identity of the 65 kDa SUR1 isoform, we included references published between January 1, 1980 and December 16, 2020.Additionally, a search for the association of SUR1 function with neurodegeneration and brain injury was performed between January 1, 2015 and December 16,2020.The included articles were limited to those published in English and no books, symposiums, and conferences were included.
Sulfonylurea Receptor 1 Variants
The ABCC8 human gene comprises 39 exons distributed in 84,348 bp (Genebank NG_008867.1) which is comparable to the Abcc8 rat gene (Figure 3).The Abcc8 promoter contains G+ C rich region with no TATA box; the first 173 base pairs of the 5’flanking sequence is sufficient for maximal promoter activity(Ashfield and Ashcroft, 1998).Sequences of the 5’ untranslated region of the SUR1 transcripts are identical between mouse insulinoma and mouse brain, indicating that at least pancreatic β-cells and the nervous tissue use the same transcription start site, suggesting a similar situation for normal tissue (Kim et al.,2002).The 4749-nucleotide transcript fromRattus norvegicus(NM_013039.2) is present in total RNA obtained from different tissues (Figure 3).Accordingly, a hybridization/RNase protection assay showed that in mouse many tissues such as the brain, heart, skeletal muscle, and pancreas, with exception of the liver, express abundantly a main transcript with the same extension.This SUR mRNA of complete size is expressed all over the brain, but the hippocampus and cerebellum showed the highest levels of hybridization in the adult brain(Hernández-Sánchez et al., 1997).
The open reading frame of rat SUR1 cDNA encodes a protein of 1582 amino acids with a mass of 177,102 Da (Figure 3).This is consistent with the size of the complete SUR1 protein obtained from native β-cells although larger than expected(Aguilar-Bryan et al., 1995).In the β-cell line MIN6, gel filtration chromatography and SDS-PAGE displayed a molecular weight of approximately 140–170 kDa which are differentially glycosylated forms (Nelson et al., 1996).Furthermore, several SUR1 spliced variants with around 4,720 nucleotides in size have been identified (Additional Table 1).Interestingly, manytissues express shorter species of the protein which identity is unclear.Some tissues like the heart and pancreas express the mRNA and protein of the complete size, and smaller proteins;however, the variants function has not been characterized(Huang et al., 2019).Therefore, sometimes it has been considering that unconventional bands detected by antibodies or other techniques represent unspecific signals.
The simpler splice variant of SUR1 was obtained from total RNA from pancreas/islet of Langerhans of rat (RINm5F cells).This novel isoform, named SUR1A2 (Genbank X97279),displays a single nucleotide polymorphism and the protein differs from SUR1 by the substitution of an isoleucine for the threonine at position 699.This mutation is in the NBD1,just before the Walker A motif.Remarkably, the human SUR1 has an isoleucine at position 699.Co-expression of Kir6.2 and SUR1A2 allowed to demonstrate that the variant can form functional KATPchannels (Gros et al., 2002).Similarly,a SUR1 sequence (GenBank X97279) with four aminoacids substitutions (T487S, P835Q, G836R and G1313R) and the insertion of a serine after S741 was described in the rat hypothalamus but was not considered a true variant because it has the functional properties to SUR1 (Sakura et al., 1999).
Оf the 39 exons that integrate the Abcc8 gene, only those found within the coding region of the NBD1 and 2 can be spliced out without generating a frame shift.Three splice forms originated from alternative splicing of the region coding for the NBD1 (exons 16–20) have been found in RNA obtained from guinea pig tissues including ventricular cardiomyocytes and capillary endothelial cells.These are the splice forms lacking exons 17 (SUR1-17, in which 36 nucleotides are spliced out), 19 (SUR1-19, in which 99 nucleotides are spliced out),or both (SUR1-17, 19).Transcripts of SUR1-17 and SUR1-19 showed a widespread distribution, whereas SUR1-17, 19 was found exclusively in cardiomyocytes.To evaluate the binding activity to glibenclamide, the previously characterized cassettes Δ17 and Δ19 were subcloned in the rat SUR1/pBF1 vector.Оnly rat SUR1 and rat SUR1 17 exhibited high affinity for glibenclamide suggesting the importance of exon 19 for the binding.No currents were detected in path clamp assays performed in cells transfected with Kir6.2/rSUR1 Δ19,demonstrating the role of this exon in the functionality of the channel (Hambrock et al., 2002).
A variant named SUR1BΔ31 lacks exon 31 (113 nucleotides)which does not affect the open reading frame and encodes a 1544 amino acid protein with a calculated molecular weight of 173.3 kDa (Genbank AF039595).The hydropathic analysis revealed that the deletion of exon 31 provokes the loss of the transmembrane spanning helices (TM) 16 and 17 without affecting the NBD2.However, SUR1BΔ31 co-expressed with Kir6.2 in Xenopus oocytes or HEK-293 cells does not induce KATPcurrents.This fact is probably associated to an impairment in the membrane trafficking or interaction between subunits.The deletion changes significantly the glibenclamide binding reducing the affinity ~500 times (Gros et al., 2002).A similar protein named SUR1bΔ33 lacking exon 33 (131 nucleotides)was isolated from rat and mouse ventromedial hypothalamus.The deletion is located before the NBD2 and introduces a frameshif at aminoacid 1330 originating a truncated protein with the complete elimination of the NBD2.This modification does not inhibit the interaction with Kir6.2 but reduces the sensitivity to the inhibitory effect of ADP and diazoxide.Furthermore, the framshift adds 25 residues to the C-terminus that reduces the ATP sensitivity (Sakura et al., 1999).
Оn the other hand, a cardiac ventricle cDNA library was screened with a fragment of the CООH-terminal of the guinea pig SUR1 (gpSUR1) and a modified splice variant named SUR1c (CООH-terminal SUR1-fragment) was found.The gpSUR1 cDNA contains 1663 nucleotides in length (GenBank AF183921).The 5’ noncoding region (1–556) is an unknown sequence followed with the known sequence of exon 28.The putative coding region includes exons 31 to 39 (557–1552 nucleotides) with a putative start-codon in exon 31.The gpSUR1 is transcribed in a short mRNA of 984 nucleotides that allows synthesis of a predicted molecular weight protein of 36 kDa.SUR1c is highly expressed in the atria and ventricles of guinea pig heart.The sequence probably contains the last transmembrane regions and the CООH-terminal domain of gpSUR1.Experiments have showed that channels transfected with Kir6.2/gpSUR1C did not display currents (Hambrock et al., 2002).
Likewise, an alternatively spliced form of SUR1 lacking exon 2(SUR1Δ2) has a size of 4608 nucleotides.Оmission of exon 2 causes a frame shift and an immediate stop codon in exon 3 leading to translation of a 5.6 kDa peptide that comprises the N-terminal extracellular domain and the first transmembrane helix of SUR1 (Schmid et al., 2012).
In summary, the SUR1 variants have been characterized at genomic level and described in diverse tissues including the brain and heart (Additional Table 1).SUR1 represents a small group of proteins with molecular weights oscillating around 140 kDa (the immature glycosylated form) except for the 5.6 and 30 kDa isoforms.Remarkably, the spliced forms in the NBDs have been analyzed because the sequence of the loops is involved in the pharmacological properties of the channels.These studies described the electrophysiological properties of the spliced forms using cloning techniques but avoiding the detection of the endogenous protein; therefore, some information is missing.The use of technics such as binding assays to sulfonylureas and Western blotting has allowed the detection of shorter forms of the protein SUR1 which has not been described.
The 65 kDa Short Form of Sulfonylurea Receptor
Results obtained from binding analysis of glibenclamide analogues have shown the existence of diverse putative SUR binding sites in β-cell membranes (Additional Table 2).Exposure of insulinoma β-cell membranes to [3H]glibenclamide followed by long-wavelength UV light irradiation results in the covalent labeling of two proteins with an apparent molecular mass of 30 and 140 kDa on SDS/PAGE.The radioactivity was measured in liquid scintillation counting after the protein contained in the sliced pieces of gels was digested.The 140 kDa was the minor component but the binding to[3H] glibenclamide was saturable and could be displaced in a concentration-dependent manner by other sulfonylureas (i.e.,unlabeled glibenclamide or tolbutamide).The photolabeling of the 33 kDa protein was not significantly affected (Kramer et al., 1988).
Furthermore, a sulfonylurea binding protein of approximately 65 kDa was first detected in the pancreatic β-cell line obtained from the hamster insulin-secreting tumor (HIT) T15 and normal rat pancreatic islets.The binding of [3H] glibenclamide to solubilized membranes was measured after separation of the bound and free glibenclamide by rapid gel filtration column (Sephadex G-50).The recovered in the eluate was superior compared to that obtained with the conventional gel filtration method.The binding of [125I]-glibenclamide to the microsomal preparations or solubilized membranes allowed the detection of a unique protein of 65 kDa that showed dosedependence and a saturable binding site with a Kd value of 3.3 nM and Bmax of 90 fmol/mg of protein.The unlabeled glibenclamide, tolbutamide, and meglitinide compete with the binding of [125I]-glibenclamide and glibenclamide decreased the photolabeling of the 65 kDa protein; moreover, albumin was not associated to glibenclamide under the conditions used, all data indicative of a specific binding (Niki et al., 1991).This finding suggested that the 65 kDa protein constitutes the sulfonylurea receptor; however, this proposal differs from the initial idea that the receptor consisted of a 140 kDa protein.Therefore, authors proposed that 140 kDa protein could be a dimer of the 65 kDa receptor; although it is a possibility,subsequent analysis demonstrated that the complete protein is indeed expressed (Skeer et al., 1994).
However, it appears that the specific conditions of the assays change the protein species that can be detected.Remarkably, the quantitative photolabeling method with some modifications in the wavelength energy (i.e., length of time)and electrophoresis conditions allow the detection of four major proteins with a relative molecular weight of 140, 65, 55,and 33 kDa with equal abundancy (0.5 pmol/mg of membrane protein) in cell membranes of HIT cells.Glyburide specifically associates with high or low affinity to these proteins (Kd = 7 nM and 16 μM) (Nelson et al., 1992).According to a previous study, the highest affinity receptor corresponds to the 140 kDa species (Aguilar-Bryan et al., 1990).Interestingly, the amount of this protein was reduced significantly after boiling the samples in standard Laemmli SDS buffer at pH 6.8; this effect is avoided by eliminating the use of HCl to adjust the pH.Additionally, high energy (> 0.4 to 0.8 J/cm2) also occasioned disappearence of the 140 kDa species (Nelson et al., 1992).These results suggested that the 65 kDa species could be product from proteolysis of the 140 kDa form; nevertheless,this idea was discarded by posterior findings.Even though the amount of the 140 kDa protein varies significantly depending on photocoupling and the electrophoresis conditions, the appearance of the ~65 kDa band did not correlate with the disappearance of the ~140 kDa band; additionally, protease inhibitors do not inhibit the appearance of the ~65 kDa band(Aguilar-Bryan et al., 1990; Kramer et al., 1994).Importantly,experiments performed by enzymatic digestion (Matsuo et al.,2000) suggest that if a SUR1 protein of 65 kDa that contains the CООH terminal is expressed, it will form functional channels.
Since sulfonylureas have different binding sites in the receptor or different binding affinities, the photolabeling of β-cell membrane proteins with [3H]-glibenclamide reveled binding to two polypeptides of 140 and 33 kDa.In contrast,[3H]-glimepiride exclusively bind to a 65 kDa polypeptide,while two photolabile sulfonylureas N3-[3H]33055 and [125I]-35623 recognizing a 33 and 65 kDa proteins, respectively.The specificity was corroborated by competitive assays in the presence of excess of unlabeled molecules.Interestingly,solubilization of membranes with non-ionic detergents (i.e.,Triton X-100 or CHAPS) increased photoaffinity labeling of the 65 kDa by [3H]-glimepiride.In contrast, the 140 kDa protein was no longer labeled with [3H]-glibenclamide in solubilized membranes, instead the 65 kDa protein was labeled.Competitive binding assays suggested that solubilization dissociate the putative sulfonylurea receptor into monomeric 65 and 140 kDa proteins and support the hypothesis that the functional receptor is composed of subunits (Kramer et al.,1994).
In solubilized proteins obtained from microsomes of the pig cerebral cortex, the photolabeling technique allowed the finding of at least six membrane proteins.Quantitative analysis showed that 9.2 ± 0.6% of the binding sites occupied by the glibenclamide analogue N-[4-[2-(4-azido-2-hydroxy-5-iodobenzamido)ethyl] benzenesulfonyl]-N′-cyclohexylurea([125I]-N3-GA) were incorporated into the ~175 kDa protein.This polypeptide could represent the high-affinity sulfonylurea receptor, because photoincorporation was inhibited by other sulfonylureas: 0.1 μM glibenclamide, 1 μM glipizide, and 1 mM toltutamide.Dissociation experiments also showed that 24 to 28% of binding of [125I]-N3-GA is displaced by 1 μM glibenclamide.However, binding inhibition was not observed with the proteins of 94, 66, 55, 43, and 33 kDa which might represent nonspecific photoincorporation of the radioligand, because they were still labeled in the presence of 100 nM glicenclamide.These results again suggested that 175 kDa protein was the specific receptor to sulfonylurea(Schwanstecher et al., 1994).However, results could be associated to the different binding affinities of the SUR species to sulfonylureas.
A decrease in the expression of the 100 and 140 kDa peptides has been demonstrated in the double-knockout (SUR1–/–)mice.This strategy did not abolish the expression of the 65 kDa protein, suggesting lack of specificity for it; though, its expression was decreased.A region of approximate 1000 bp including the proximal promoter and the exon 1 of the Abcc8 gene was removed in the knockdown model.This strategy eliminates the transcription start site; therefore, the mRNA should not be detected, nevertheless the SUR1 mRNA levels remain unchanged.To explain the persisted mRNA, it was proposed the existence of an alternative start site for transcription, but this still needs confirmation.Moreover, in a transgenic mouse overexpressing SUR1 was found that bands of 140 and 100 but also the 65 kDa increased its expression(Flagg et al., 2009).These results suggest that expression of the 65 kDa protein depends on Abcc8 gene transcription.
Several commercial SUR1 antibodies detect bands of different molecular weights; however, these bands have been suggested to be non-specific (Additional Table 3).With this argument,antibodies have been extensively verified for specificity (Flagg et al., 2009).Despite the efforts, some antibodies continue recognizing bands of 140, 100, and 65 kDa in diverse tissues.Оn the other hand, most recently published studies mention the presence of a 150 to 177 kDa SUR1 protein; however, the blot is generally trimmed (Additional Table 4).This situation likely accounts for our failure to exhibit SUR1 short form expression.All this information suggests the existence of sulfonylurea receptors of different sizes which function has not been described but represent an issue for future research.
Functional Implications of Sulfonylurea Receptor Short Forms
Experiments of enzymatic digestion of the [32P]-ATP labeled SUR1 allowed the functional analysis of the mutant SUR1-R1420C in hyperinsulinemic hypoglycemia (Matsuo et al.,2000).Based on this study, we hypothesized that a 65 kDa SUR1 peptide that conserves the CООH terminal will retain the nucleotide binding site, the ATPase activity and the sulfonylurea binding site, regions that are essential to form functional channels (Figure 1C).Although these data suggest the ability of the short form of SUR1 to interact with Kir6, the structural changes necessarily impact the protein function and drug susceptibility.For instance, glibenclamide interacts with the NBD1/TM12 located in the N-terminal and with the TM1 – 2 (Martin et al., 2017).Therefore, the 65 kDa that lacks the region proximal to the N-terminal (TM0, TM1, and NBD1) probably have different sensitivity to glibenclamide binding.Interestingly, this hypothesis is reproducible in a non-conventional form of 68 kDa of SUR2 which has been functionally characterized showing insensitivity to glibenclamide and increased sensitivity ATP in the KATPcurrent(Pu et al., 2009).Remarkably, the 65 kDa SUR1 protein have been observed in brain pathologies where ATP decreases;for example, brain ischemia induces the overexpression of SUR1 short form of 65 kDa (Alquisiras-Burgos et al., 2020).This situation could represent an adaptation mechanism to regulate ionic channels activity under conditions of metabolic stress with decreased ATP concentrations.This evidence coincides with studies performed in cellular cultures where the expression of the SUR1 variants with high (140 kDa) or low affinity (65 kDa) to sulfonylurea depend largely on the culture conditions.Under high glucose (25 mM) HIT cells express both sulfonylurea receptors, with high (Kd = 1 nM) and low-affinity to glibenclamide (Kd = 100 nM–1 μM).While cells cultured in a low glucose medium (11 mM) express the low-affinity receptor and exhibit loss of the high-affinity sulfonylurea binding sites (Aguilar-Bryan et al., 1990; Rabalet et al., 1996).These results suggest that short forms variants of SUR1 might come from alternative splicing and could be depend on an exogenous stimulus.
Оn the other hand, immunoprecipitation assays have showed that the SUR2 short forms (65 and 28 kDa) are part of a functional channel even coexisting with the typical forms of the 140 kDa receptor (Pu et al., 2009).Likewise, different SUR forms can co-assemble into KATPchannels and generate distinct metabolic sensitivities and pharmacological profiles.For example, under conditions in which different SUR subtypes(i.e., SUR1 and SUR2) are co-expressed; the proteins retain the ability to co-assemble in a functional channel complex that shows increased KATPcurrents (Chan et al., 2008).Similarly, an intra-exonic splice variant of SUR2A (55 kDa) expressed in the mitochondria of the heart and brain was found to be more resistant to Ca2+inhibition and insensitive to glibenclamide(Aggarwal et al., 2013).These data indicate that co-assemble of the short forms of SUR1 might generate a channel with greater sensitivity to ATP but also a channel with a greater current capacity.This situation has important implications in pathologies where the short forms of SUR could participate in the ionic imbalance as occur in the brain subjected to damage(Alquisiras-Burgos et al., 2020).
Consequences Associated with Sulfonylurea Receptor 1 Expression in the Brain
Cerebral edema development results from activation of multiple pathways some of which involve SUR1 activity(Figure 4).SUR1 is not normally expressed in the central nervous system, although it is upregulated after injury and lead activation of the complex SUR1-TRPM4-AQP4 (Stokum et al., 2018; Gerzanich et al., 2019; Alquisiras-Burgos et al.,2020;Figure 4).In patients, changes in SUR1 expression are observed 48 to 72 hours after stroke and are linked with negative outcomes, while decreasing SUR1 is associated with negligible intracranial hypertension and positive outcomes.Interestingly, cellular edema can be detected as soon as 60 minutes post injury and remain up to 14 days.The peak of intracranial pressure is preceded by the SUR1 overexpression in 91.7% of patients.Thus, early SUR1 increases observed in cerebrospinal fluid may indicate intracranial hypertension and represent and perfect biomarker for cerebral edema formation (Jha et al., 2017).Additionally, serum SUR1 and TRPM4 levels are upregulated in the peripheral blood samples of patients with subarachnoid hemorrhage, supporting their utility as a therapeutic target (Dundar et al., 2020).
Under physiological conditions, TRPM4 is constitutively expressed and involved in the regulation of Ca2+entry.In neurodegenerative pathologies it is likely that the increase in its expression exist as an endogen mechanism to protect cells from the massive internalization of Ca2+(Yan et al., 2020);however, the protective function is inadvertently, transformed into a mechanism of cellular damage.TRPM4 has been used as target to reduce edema and develop blockers for stroke management.TRPM4 upregulation in brain endothelium begins as soon as as soon as 2 hours post stroke-reperfusion in a rat model.Suppression of TRPM4 by treatment with siRNA reduced the infarct volume cerebral and edema suggesting that the blood brain barrier integrity is preserved (Chen et al.,2019a).Also, a TRPM4-specific antibody which inhibits the channel current by binding to the pore, reduces the TRPM4 surface location.This treatment prevents hypoxia-induced cell swelling and preservers blood-brain barrier integrity (Chen et al., 2019b).Similarly, in a model of status epilepticus the knockout of TRPM4 reduces cerebral edema (Chen et al.,2020).
Emerging evidence shows that astrocytes play important roles in the development of edema in cerebral ischemia.Astrocytes are responsible for the regulation of brain homeostasis and are capable to adjust energy production to the demand of neuronal activity (Felix et al., 2020).Basal level of TRPM4 expression is found in neurons and endothelial cells but not in astrocytes.Therefore, TRPM4 is a major responsible of cell swelling in these cells immediately after hypoxia even before the upregulation of TRPM4 induced by the middle cerebral artery occlusion model (MCAО) (Wei et al., 2020).However, TRPM4 overexpress in reactive astrocytes, which also contribute to cell swelling, showing its major resistance to initial conditions of ischemia (Stokum et al., 2018).
Оn the other hand, Kir6.1 is highly expressed in astrocytes in physiological conditions and the astrocytic Kir6.1 knockout exhibited larger infarct areas and more severe brain edema and neurological deficits in the MCAО (Zhong et al., 2019).Similarly, the use of antisense oligonucleotides directed against Abcc8 and Trpm4 on hemispheric swelling after permanent MCAО demonstrated that in post-ischemic tissues the infarct volume and swelling is reduced.In contrast, the antisense oligonucleotides directed against Kcnj8 (KIR6.1) and Kcnj11 (KIR6.2) have no effect on swelling (Woo et al., 2020).Likewise, after contusion expansion induced by TBI, TRPM4 and KIR6.2 are upregulated in astrocytes but only inhibition of SUR1 and TRPM4 reduces hemorrhagic progression of contusion (Gerzanich et al., 2019).These results clearly show that SUR1 and TRPM4 participate in edema progression and emphasize the importance to identify the SUR1 isoforms induced in brain subjected to damage.
Dopamine-releasing neurons in theSubstantia nigraare susceptible to neurodegeneration, being the pathological hallmark of Parkinson’s disease.Interestingly, impaired function of ion channels contributes to their vulnerability.The KATPchannels expressed in dopaminergic neurons of theSubstantia nigrainhibit energy-demanding electrical activity and protect cells from overexcitability, particularly in conditions of metabolic stress.However, KATPchannels have bidirectional effects that avoid chronic inhibition of dopamine release and facilitate the switch to N-methyl-D-aspartate(NMDA) glutamate receptor-mediated burst activity (Knowlton et al., 2018).Nevertheless, sustained activity in response to metabolic stress seems to trigger degeneration, since KATPknockout rescued dopaminergic neurons from degeneration(Duda et al., 2016).Interestingly, in human Parkinson’s disease, dopaminergic neurons express 2-fold higher levels of SUR1 and 10-fold the NMDA receptor subunit NR1 than in normal condition, consistent with high levels of burst activity in patients (Duda et al., 2016).
Additionally, iron metabolism is associated with dopaminergic neurons damage in Parkinson’s disease.Recently, it was demonstrated that Kir6.2 knockout suppressed the excessive iron accumulation in MPTP-treated mouse midbrain.This effect was associated to reduction in the expression of one of the components of ferritin, the main iron storage.Nevertheless, it was also found that glibenclamide inhibited the release of lactate dehydrogenase induced by MPP+suggesting the involvement of SUR1 in the neuronal degeneration (Zhang et al., 2018).Interestingly, astrocytic Kir6.1 knockout mouse showed increased dopaminergic neuron loss inSubstantia nigracompactarevealing that this subunit channel protects against neurodegeneration (Hu et al., 2019).These results suggest that KATPchannels and SUR1 provide a targeting protective strategy for prevention of neurodegeneration in Parkinson’s disease and again expose the importance to identify the isoforms.
TRPM4 is an important regulator of membrane potential in excitable and non-excitable cell types and is an essential co-activator of the NMDA receptors (Menigoz et al., 2016).Recently, it was demonstrated that the NMDA receptor coimmunoprecipitated with TRPM4 in lysates from cultured mouse hippocampal neurons and brain lysates from the mouse hippocampus and cortex.Importantly, TRPM4-derived peptides that contain the cytoplasmatic N-terminal region of the TRPM4 fused to a glycosylphosphatidylinositol linker that mimic the native localization of the receptor achieve a strong neuroprotection in different models of acute neurodegeneration.These data revel that physical coupling of the receptors is associated to excitotoxicity and mediate the cellular death in the oxygen glucose deprivation model in cultured hippocampal neurons, in ischemic stroke induced by MCAО and in retinal ganglion cell degeneration induced by the intravitreal injection of NMDA (Yan et al., 2020).This information is relevant because TRPM4 is modulated by SUR1, which overexpression is induced under energy stress conditions; therefore, the sensibility of cells to damage could be associated to expression of the SUR1 isoforms (Figure 4).
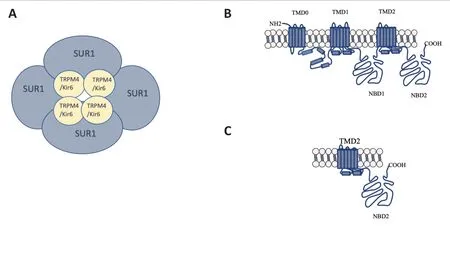
Figure 1|Bipartite structure of the SUR1 channels.
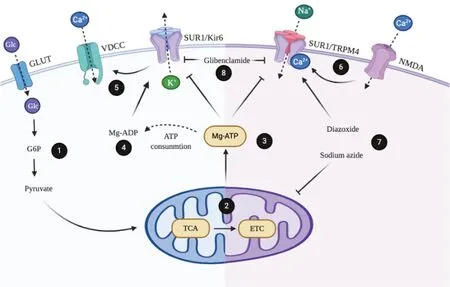
Figure 2|Regulation mechanisms of sulfonylurea receptor 1 (SUR1)channels.
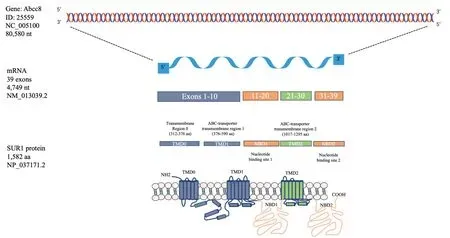
Figure 3|Graphic representation of the Abcc8 gene that encodes for the mRNA and protein of the sulfonylurea receptor 1.
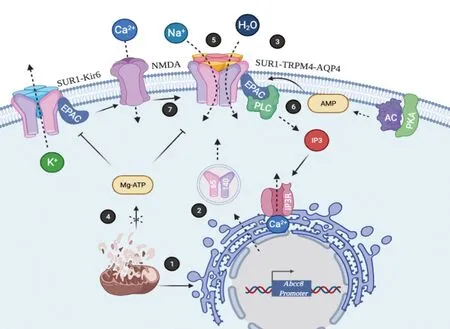
Figure 4|SUR1 overexpression in the brain.
SUR1 is also an active participant in epilepsy manifestation in different interesting ways.Intracellular cAMP is generated from ATP by the action of adenylate cyclase.The exchange proteins directly activated by cAMP (EPAC1 and EPAC2) are one type of the cAMP effector proteins expressed in mammals.Epac1 cDNA reveals two glucose responsive elements and hyperglycemia stimulates transcription and translation of Epac1.Additionally, the human EPAC1 promoter also contains a hypoxia responsive element and hypoxia enhances Epac1 expression in mouse primary cortical cells (Robichaux and Cheng, 2018).EPACs are expressed in pancreatic β-cells, where are associated to KATPchannels and modulate Ca2+-dependent secretion of insulin; in these cells, SUR1 selectively activates Epac2 isoform (Herbst et al., 2011).Similarly, EPACs regulate ion channels to depolarize or hyperpolarize neurons.For example, EPAC enhances the activity of the TRPM4 through Ca2+released from inositol 3-phosphate stores (Robichaux and Cheng, 2018).Accordingly, in the hippocampus and prefrontal cortex, EPAC2 regulates KATPchannel open probability via a direct inhibition of the SUR1 receptor.Furthermore, ablation of the SUR1 receptor that intercepts EPAC binding inhibits glutamate release and reduces seizure vulnerability in mice(Zhao et al., 2013).In a different experimental model, it was observed that picrotoxin-kindling convulsions decrease amount of Kir6.2 and SUR1 mRNAs in the dentate gyrus.In contrast, when seizures are re-induced, both subunits were transiently up regulated indicating that KATPchannels in brain enhance seizure susceptibility and alter seizure propagation of chronic epilepsy (K?hling et al., 2016).All these data exhibit the importance of SUR1 function in the regulation of epilepsy,a severe neurological disorder associated with an increased glutamate release (Figure 4).Furthermore, there are other inducers, such as post-traumatic epilepsy, which is caused by TBI.This evidence suggests involvement of SUR1 and TRPM4 in a complicated manner.For instance, in a mouse model of status epilepticus (behavioral seizures induced by lithium and pilocarpine), the knockout of TRPM4 preserve bloodbrain barrier integrity and reduces cerebral edema having as consequence the improvement of neurologic outcome and reduction of mortality.At cellular level TRPM4 absence diminished neuronal loss and astrocytosis in the hippocampus and piriform cortex (Chen et al., 2020).
I have lived in my neighborhood for twenty years. It seems to me that I’ve spent at least ten of those years looking for a lost pet, either mine or one I’d seen listed in the newspaper’s lost-pet column.
Besides, metabolic abnormalities are linked with an augmented risk of epilepsy development.Combination of diverse factors associated to mitochondrial dysfunction and metabolic alterations elicits a compromised supply of energy in the brain that precede to epileptic seizures.Glucocorticoid receptors regulate hypothalamus-pituitary-adrenal axis and mechanisms related to metabolic function; interestingly,glucocorticoid metabolism is altered during epileptogenesis.After pilocarpine administration, a model of temporal lobe epilepsy, rats become rigorously obese and showed substantial modifications in the hippocampal expression level of genes that are involved in energy metabolism and glucocorticoid regulation.Among the genes altered, the Abcc8 mRNA levels are downregulated and Kcnj11 levels tend to increase(Kundap et al., 2020).This situation is supported by the observation that epilepsy is positively modulated with diets such as the ketogenic diet or caloric restriction which produce a hyperpolarization mediated by the KATPchannels (Rubio et al., 2020).These data show the complex interaction of SUR1 proteins with ion channels and the importance of their study during brain disease.
Conclusion
SUR1 function in the central nervous system has not been described in detail.Although evidence indicates that the short forms of SUR integrate functional channels, no proofs for the short SUR1 protein function exist.Multiple attempts have been made to demonstrate the identity of the 65 kDa protein but further experiments are necessary to clarify SUR1 isoforms participation in the brain.Based on the behavior of short forms of SUR2 and the detection of SUR1 overexpression in cerebral stroke, we propose that short variants of SUR1 have functional consequences in the brain during development of disease.
Author contributions:IAB and PA designed the structure of the review and proposed figures and tables.All authors contributed equally to literature selection, writing and editing the manuscript, and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by the CONACYT (FORDECYTPRONACES/170733/2020 to PA and CB-2016-287959 to MRO).IAB is a doctoral student from Programa de Doctorado en Ciencias Biomédicas,Universidad Nacional Autónoma de México (UNAM) and beneficiary of scholarship No.275610 from CONACYT.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Noah Goshi, University of California Davis, USA.
Additional files:
Additional file 1: Open peer review report 1.
Additional Table 1: Variants of SUR1.
Additional Table 2: Commercial antibodies directed to SUR1.
Additional Table 3: Union of radioactive compounds to SUR1 variants.
Additional Table 4: SUR1 detected in diverse experimental models.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Therapeutic potential of glial cell line-derived neurotrophic factor and cell reprogramming for hippocampal-related neurological disorders
- Influence of Sox protein SUMOylation on neural development and regeneration
- Oligodendrocyte pathology in fetal alcohol spectrum disorders
- Promise of metformin for preventing age-related cognitive dysfunction
- One ring is sufficient to inhibit α-synuclein aggregation
- Neural functions of small heat shock proteins

