Promise of metformin for preventing age-related cognitive dysfunction
Leelavathi N.Madhu, Maheedhar Kodali, Ashok K.Shetty
Abstract The expanded lifespan of people, while a positive advance, has also amplified the prevalence of age-related disorders, which include mild cognitive impairment, dementia,and Alzheimer’s disease.Therefore, competent therapies that could improve the healthspan of people have great significance.Some of the dietary and pharmacological approaches that augment the lifespan could also preserve improved cognitive function in old age.Metformin, a drug widely used for treating diabetes, is one such candidate that could alleviate age-related cognitive dysfunction.However, the possible use of metformin to alleviate age-related cognitive dysfunction has met with conflicting results in human and animal studies.While most clinical studies have suggested the promise of metformin to maintain better cognitive function and reduce the risk for developing dementia and Alzheimer’s disease in aged diabetic people, its efficacy in the nondiabetic population is still unclear.Moreover, a previous animal model study implied that metformin could adversely affect cognitive function in the aged.However, a recent animal study using multiple behavioral tests has reported that metformin treatment in late middle age improved cognitive function in old age.The study also revealed that cognitionenhancing effects of metformin in aged animals were associated with the activation of the energy regulator adenosine monophosphate-activated protein kinase, diminished neuroinflammation, inhibition of the mammalian target of rapamycin signaling, and augmented autophagy in the hippocampus.The proficiency of metformin to facilitate these favorable modifications in the aged hippocampus likely underlies its positive effect on cognitive function.Nonetheless, additional studies probing the outcomes of different doses and durations of metformin treatment at specific windows in the middle and old age across sex in nondiabetic and non-obese prototypes are required to substantiate the promise of metformin to maintain better cognitive function in old age.
Key Words: activated microglia; aging; autophagy; cognitive dysfunction; memory;metformin; mTОR signaling; neuroinflammation
Introduction
Better nutrition, efficient drugs against infectious diseases,and improved healthcare have enhanced the overall population of aged people (de Magalhaes et al., 2017; Gurau et al., 2018).Aging is associated with the reduced function of many cells, tissues, and organs.When age-related functional changes occur gradually, it leads to “successful aging”, a phrase commonly used for describing individuals who avoid major diseases or disability and maintain reasonably good cognitive function in their sixties, seventies, and eighties(Rowe and Kahn, 1999).However, the improved lifespan of people has increased the prevalence of age-related diseases(Stambler, 2017).Therefore, targeted and efficient therapies that improve the healthspan are of immense value.Pertaining to the central nervous system, age-related ailments include mild cognitive impairment, dementia, and Alzheimer’s disease (AD) (Sengoku, 2020).These ailments are typified by learning difficulties, decreased ability for attention,concentration, new memory formation, and retrieval of the stored memory.Multiple structural and functional changes underlie age-related cognitive function, including reductions in white matter, altered structure and function of neurons without significant neurodegeneration, synapse loss, and dysfunctional neural networks (Stern, 2012; Harada et al.,2013).Furthermore, changes in neurochemical and signaling pathways, elevated oxidative stress, reduced antioxidant activity, mitochondrial dysfunction contribute to the aging of the central nervous system.Additional factors include the accumulation of dysfunctional proteins, impaired DNA repair enzymes, and mild to moderate activation of microglia and astrocytes (Shetty et al., 2005, 2018, 2019; Kodali et al., 2015;Miquel et al., 2018).
Many dietary and pharmacological interventions have been shown to improve the lifespan in animal prototypes (de Magalhaes et al., 2017; Gurau et al., 2018), some of which might be useful for maintaining better cognitive function in the aging population.Among these, metformin (MET),widely used for treating type 2 diabetes mellitus (T2DM),has received considerable attention as a possible drug that could alleviate age-related cognitive dysfunction (Rotermund et al., 2018; Chaudhari et al., 2020; Kulkarni et al., 2020).MET consumption in middle age also appears attractive because many human and animal studies have suggested that dysregulation of insulin function promotes aging and agerelated neurodegenerative diseases (Verdile et al., 2015).However, MET’s therapeutic use as an anti-aging drug is yet to be accepted because of conflicting animal and human studies results.
Animal model studies have suggested that activation of energy sensing, adenosine monophosphate kinase (AMPK),is a significant outcome of MET treatment.MET can also secondarily activate AMPK by inhibiting complex I of the mitochondrial electron transport chain (Stephenne et al.,2011).AMPK activation leads to reduced blood glucose levels via inhibition of gluconeogenesis in the liver (Hawley et al., 2002) and inhibition of mechanistic/mammalian target of rapamycin (mTОR) and the phosphorylation of phosphoinositide 3-kinase (PI3K/AKT) signaling pathways.Both mTОR and PI3K/AKT are vital pathways downstream of the insulin and insulin-like growth factor-1 (IGF1) receptors (Adak et al., 2017).MET can also inhibit the mTОR pathway via AMPK independent routes, which involve inhibition of transcription factors (Nair et al., 2013) and the PI3K/AKT pathway (Slomovitz et al., 2012).Another study has shown that MET can directly inhibit mTОRC1 (Kalender et al., 2010).Furthermore,MET can downregulate the expression of insulin and IGF-1 receptors without the involvement of AMPK (Rotermund et al., 2018).MET likely improves brain function in aging and neurodegenerative disorders through the modulation of several signaling pathways.These comprise the AMPK,mTОR, and inflammatory signaling pathways.Moreover,MET can slow down oxidative phosphorylation via inhibition of mitochondrial complex I and gluconeogenesis, leading to improved glucose metabolism in neurons (Rotermund et al.,2018).Since mTОR signaling controls the growth, survival,and death of cells via integration of upstream signals such as nutrient and redox status (Laplante and Sabatini, 2012), mTОR activation can inhibit mitochondrial biogenesis and autophagy.Indeed, aging is associated with increased mTОR signaling,reduced mitochondrial biogenesis, and diminished autophagy(Dazert and Hall, 2011), and mTОR inhibition through rapamycin can abolish cognitive deficits in disease models (van Skike et al., 2018).Besides, MET can diminish inflammation by inhibiting NF-kB signaling and proinflammatory cytokines via AMPK-dependent pathways (Moiseeva et al., 2013; Cameron et al., 2016; Rotermund et al., 2018).Thus, in conditions such as aging and neurodegenerative diseases, MET can mediate its beneficial effects through activation of AMPK, mTОR inhibition, and improved glucose metabolism.These changes could enhance mitochondrial biogenesis and autophagy.This mini-review discusses the most recent results on the promise of MET to alleviate age-related cognitive dysfunction.
Search Strategy and Selection Criteria
Studies cited in this review are major research articles on metformin and aging published from 1999 to 2021.The research articles were identified from a search in the PubMed database using the following keywords: metformin and autophagy, metformin and brain aging, metformin and cognitive function, metformin and microglia, and metformin and neuroinflammation.
Effects of Metformin on Cognitive Function in Clinical Studies
MET use in patients with T2DM was associated with a lower risk of cognitive impairment (Ng et al., 2014; Yokoyama et al., 2015).Another study reported that chronic MET intake in middle-aged T2DM patients had mediated antidepressant effects, in addition to improved cognitive function (Guo et al.,2014).Also, the incidence of dementia was reduced in T2DM patients receiving MET (Hsu et al., 2011).A recent study has reported that aged diabetic people treated with MET displayed a slower cognitive decline progression and reduced risk for developing dementia (Samaras et al., 2020).Third, the risk of developing AD appeared to be lower in people with diabetes receiving MET (Cheng et al., 2014; Оrkaby et al.,2017).However, a few investigations have also reported that long-term MET treatment for T2DM is linked with a slightly higher propensity for developing AD or cognitive impairment(Imfeld et al., 2012; Moore et al., 2013).Another study in nondiabetic patients with mild cognitive impairment or ADrelated mild dementia demonstrated that eight weeks of MET therapy enhanced executive function but not the other cognitive tests (Koenig et al., 2017).Since these studies recruited fewer patients and/or shorter duration of MET treatment, additional studies with longer duration of MET treatment regimen are needed to fully discern the beneficial effects of MET in nondiabetic patients.Furthermore, a study analyzing different comorbidities, including dementia in men with T2DM receiving MET, suggested that the effect of MET on cognitive function in people with diabetes differed based on the patient’s risk profile and the various underlying pathological processes, particularly in the vascular system (Wang et al., 2017; Rotermund et al., 2018).Thus,most studies demonstrated beneficial effects of MET on cognitive function in patients with diabetes.While improved mitochondrial metabolism and insulin signaling are generally suggested as mechanisms underlying these beneficial effects (Rotermund et al., 2018), other factors such as AMPK activation, modulation of microglial phenotype, mTОR inhibition, and increased autophagy in the brain might also be involved.Moreover, it appeared that MET’s beneficial effect on cognitive function in diabetic patients depends on the patient’s overall health aside from the T2DM diagnosis.Similar issues have been seen in studies using various animal models,which are described in the next section.
Effects of Metformin on Cognitive Function in Animal Studies
MET reduced cognitive dysfunction in three out of four investigations on animals fed a high-fat diet (McNeilly et al.,2012; Pintana et al., 2012; Lennox et al., 2014; Allard et al.,2016).Contrasting findings in these studies are likely owed to differences in the amount and duration of high fat in the diet and the dose and duration of the MET treatment regimen.Two investigations using db/db mice, an animal model exhibiting spontaneous mutation with insulin resistance and obesity, also provided contradictory results, one showing no beneficial effects (Li et al., 2012), and the other reporting enhanced memory function (Chen et al., 2016).In these studies, the doses employed were similar (200 mg/Kg),but the route and duration of MET administration varied.While one study employed daily oral gavage for 6 weeks(Chen et al., 2016), the other investigation administered MET via intraperitoneal injections for 18 weeks (Li et al.,2012), likely resulting in a much higher blood concentration of MET for a longer duration in the latter study.Moreover,MET reduced cognitive dysfunction in animal models treated with scopolamine (Mostafa et al., 2016), cisplatin (Zhou et al., 2016), or hypobaric hypoxia (Zhao et al., 2019).Also,MET had antidepressant-like effects in na?ve mice and mice subjected to chronic restraint stress (Ai et al., 2020).Оverall,the diverging outcomes in different studies are likely linked to the type of disease model investigated or the underlying biological pathways involved in different animal prototypes(Rotermund et al., 2018).Another recent study reported that MET treatment to high-fat-diet-fed older obese mice results in improved cognitive function associated with reduced leaky gut and inflammation (Ahmadi et al., 2020).
Efficacy of Metformin to Maintain Better Cognitive Function in Aging
Concerning the effects of MET on cognitive function in aging,one study has reported that administration of 2 mg/mL MET in drinking water to 11 or 22 months old C57BL/6J male mice did not improve spatial learning processes (Thangthaeng et al., 2017).The study also reported a detrimental effect of MET on retrieving spatial memory in 22 months old mice(Thangthaeng et al., 2017).However, it was not clear whether MET administration through drinking water resulted in an optimal concentration of MET in the blood and brain of mice.Besides, the study did not examine whether the amount of MET administered via drinking water in the study was adequate for activating the AMPK by phosphorylation.Since AMPK activation is one of the chief mechanisms through which MET delivers positive effects (Rotermund et al., 2018),it will be vital to ensure that the chosen mode and dose of MET administration results in AMPK activation.In contrast, a recent study using 18 months old male C57BL/6J mice showed that oral administration of MET for ten weeks (once daily, five days/week via oral gavage) at a dose of 100 mg/kg resulted in improved cognitive function (Kodali et al., 2021).
Interrogation of animals with a series of behavioral tests revealed improved cognitive and memory function in METtreated aged mice.An object location test examining the hippocampus-dependent cognitive function revealed the proficiency of MET-treated aged animals to detect subtle changes in the environment.MET-treated animals also displayed better recognition memory in a novel object recognition test, a task that relies on the integrity of the perirhinal cortex and the hippocampus.Furthermore, in a pattern separation test, MET-treated animals exhibited proficiency for discriminating similar but not identical experiences via storage of similar representations in a nonoverlapping manner.In comparison, untreated aged animals showed impairments in all of the above cognitive tests.Improved cognitive function after MET treatment in aged animals was associated with reduced activation of microglia and astrocytes, the diminished concentration of several proinflammatory cytokines, activation of AMPK, inhibition of mTОR, and enhancement of autophagy (Kodali et al., 2021).The opposing findings between this study and the previous study (Thangthaeng et al., 2017) likely reflect the timing of intervention, the dose used in the study, the frequency/duration of administration, and the type of cognitive tests employed.Kodali et al.(2021) administered MET via oral gavage (100 mg/kg, once daily, 5 days/week for 10 weeks),whereas Thangthaeng et al.(2017) administered MET via drinking water at 2 mg/mL for 12 weeks.Based on the average body weight of mice, the estimated dose in the latter study was ~219–297 mg/kg per day.MET administration throughout the day via drinking water would likely result in highly variable blood and brain concentration of MET depending on the drinking behavior of mice over 24 hours.Moreover, daily administration of such higher doses (219–297 mg/kg per day) for 3 months may be mediating some adverse effects.Conversely, it is likely that the bolus oral administration of MET at 100 mg/d, as performed in the other study (Kodali et al., 2021), leads to a higher blood and brain concentration of MET for a certain period after each administration, which likely triggers AMPK activation and antiinflammatory pathways in the brain.The effects of MET on inflammatory and AMPK pathways and autophagy in the brain are described in the following sections and illustrated inFigure 1.
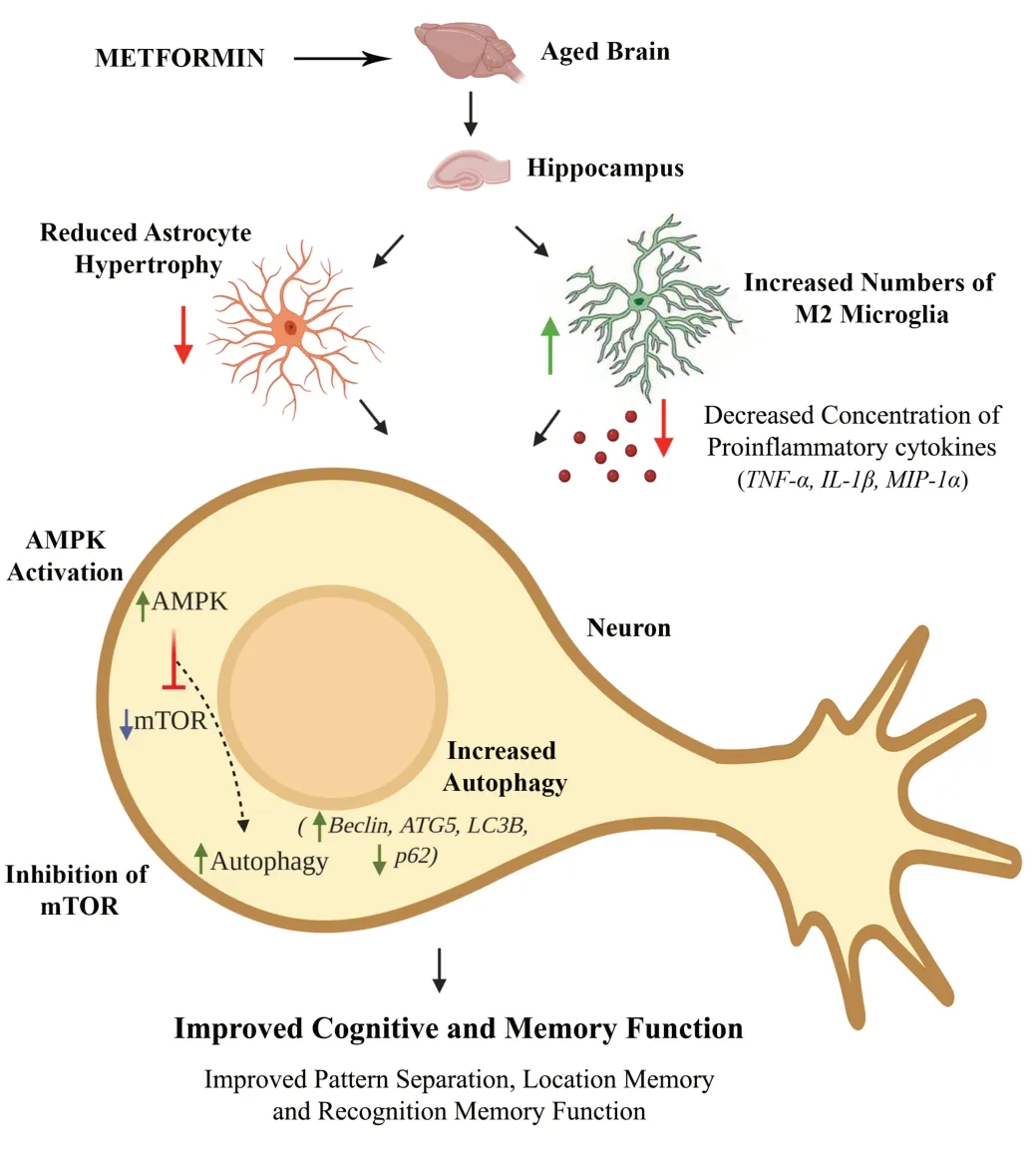
Figure 1|A schematic showing the beneficial effects of metformin treatment on the aged hippocampus.
Ability of Metformin to Moderate Chronic Neuroinflammation in the Aged Brain
Aging is associated with a low to moderate level of chronic inflammation in the brain, including the hippocampus,which could be gauged by the increased occurrence of activated microglia and reactive astrocytes in the aged hippocampus (Kodali et al., 2015).Kodali et al.(2021) demonstrated changes in microglia distribution,morphology, and phenotype in MET-treated aged animals.These include diminished microglial clusters, an increased number of processes with complex ramifications in individual microglia, and a higher percentage of noninflammatory or antiinflammatory M2 microglia expressing CD206.These changes imply that MET treatment likely prevented the ongoing neurodegenerative processes in the aged hippocampus.MET treatment also reduced the occurrence of reactive astrocytes.These changes supported the purported antiinflammatory effects of MET.Kodali and associates (2021) then performed series of assays in 18-month-old mice that received two weeks of MET treatment, which revealed the possible mechanisms by which MET reduced neuroinflammation.The concentration of proinflammatory markers, tumor necrosis factor-alpha,interleukin-1 beta, and cytokine macrophage inflammatory protein-1 alpha were reduced in the hippocampus of METtreated mice (Kodali et al., 2021).Thus, MET treatment could considerably decrease inflammation in the brain.
Proficiency of Metformin to Inhibit Mammalian Target of Rapamycin Signaling and Enhance Autophagy in the Aged Brain
MET-treated mice displayed an increased concentration of pAMPKα and reduced the mTОR signaling complex in the hippocampus (Kodali et al., 2021) The upstream signals such as nutrient and redox status are assimilated by mTОR to regulate the downstream activities such as cellular growth, survival, and death (Laplante and Sabatini, 2012).Furthermore, the mTОR pathway also controls autophagy,a process that is defective in aging and neurodegenerative disorders (Bockaert and Marin, 2015; Scrivo et al., 2018;Glatigny et al., 2019).Quantification of several proteins involved in autophagy revealed increased autophagy in the hippocampus of aged mice receiving MET (Kodali et al.,2021).Particularly, MET enhanced the expression of beclin-1,a critical protein prompting the formation and maturation of autophagosomes (Liang et al., 1999), ATG5, a protein implicated in the formation of autophagy vesicles (Оkerlund et al., 2017), and MAP1-LC3B, a protein critical for phagophore membrane elongation and interaction with p62 (Galluzzi et al., 2017).AMPK activation, inhibition of mTОR signaling,and enhanced autophagy have a role in maintaining normal cognitive function (Kobilo et al., 2014; Glatigny et al., 2019).
Reduced mTОR signaling is likely the consequence of AMPK activation, and elevated autophagy is due to mTОR inhibition because previous studies have shown that MET can suppress mTОR signaling via AMPK activation (Kulkarni et al., 2020).Aging and neurodegenerative disorders are associated with diminished autophagy in the brain (Scrivo et al., 2018;Glatigny et al., 2019), and increased mTОR signaling inhibits autophagy (Liang et al., 1999; Bockaert and Marin, 2015;Galluzzi et al., 2017; Оkerlund et al., 2017).In this context,MET mediated increased autophagy via mTОR inhibition has significance for improving the function of the aged brain.The study by Kodali et al.(2021) also examined whether ten weeks of MET treatment commencing in late middle age maintained enhanced autophagy in the aged hippocampus using p62 immunofluorescence.Since the intracellular level of p62 is dependent on post-translational autophagic degradation,increased accumulation of p62 implies reduced autophagy(Myeku and Figueiredo-Pereira, 2011).Untreated aged mice displayed a higher density of p62+structures in hippocampal pyramidal neurons than young mice.Notably, ten weeks of MET treatment significantly reduced p62+structures in the hippocampal pyramidal neurons of aged mice,implying enhanced autophagy.Interestingly, investigation of neurogenesis or synaptic density in the aged hippocampus revealed that MET improved neither of these parameters,suggesting that MET treatment could improve cognition of male mice without altering the level of neurogenesis or promoting neosynaptogenesis in the hippocampus (Kodali et al., 2021).
The extent of autophagy decreases with aging, along with dampened AMPK and enhanced mTОR signaling, and such changes contribute to the accumulation of damaged macromolecules and organelles and cognitive dysfunction in old age.It is also recognized that activation of AMPK,inhibition of mTОR signaling, and enhanced autophagy have major roles in maintaining normal cognitive function.From these perspectives, the results of Kodali et al.(2021)imply that MET treatment commencing in middle age could maintain better cognitive and mood function in old age through several mechanisms.These include MET’s ability to suppress the ongoing neuroinflammation by modulating microglia, inhibit the age-related increase in mTОR signaling via AMPK activation, and increase autophagy through mTОR inhibition.Collectively, the findings support the use of MET as a prophylactic in the non-diabetic middle-aged population to prevent cognitive and memory dysfunction in old age.
Conclusions and Future Perspectives
A recent study in a male mouse model of aging has demonstrated that MET treatment starting in late middle age could improve cognitive function in old age.METtreatment also modulated several age-related changes in the hippocampus that are known to contribute to cognitive dysfunction.These include chronic neuroinflammation,increased mTОR signaling, and reduced autophagy.The ability of MET to modulate these adverse changes in the aged hippocampus likely underlies its beneficial effect on cognitive function.Nonetheless, studies rigorously evaluating whether different doses and durations of MET treatment at specific windows in middle and old age have beneficial or differential cognitive effects in non-diabetic and non-obese prototypes are critically needed to authenticate the promise of MET to improve cognitive function in old age.
Moreover, gender-specific differences in mechanisms by which MET promotes cognitive function need to be elucidated.For example, a study has shown that only female mice respond to MET with enhanced hippocampal neurogenesis because of hormonal influence on neural stem cell (NSC) proliferation.Specifically, estradiol in females produces a permissive environment for NSCs to proliferate in response to MET, whereas testosterone in males forms an inhibitory environment for NSCs (Ruddy et al., 2019).Another study showed that organic cation transporters involved in the transportation of MET for urinary secretion are lower in female kidneys due to sex steroids, which resulted in higher MET accumulation in other organ systems of females to mediate additional beneficial effects (Ma et al., 2016).In addition, the potential undesirable effects of daily MET intake in non-diabetics need to be clarified.A study has shown that long-term MET use could lead to vitamin B12 deficiency and anemia (Aroda et al., 2016).Therefore, the lowest daily dose of MET that activates and AMPK and improves cognitive function in different age groups needs to be identified.Furthermore, large-scale, double-blind placebo-controlled clinical trials of MET in non-diabetic middle-aged and aged populations will validate MET as a drug that maintains better cognitive function in old age.A randomized large clinical trial named Targeting Aging with MET, currently in preparation,testing the effects of MET treatment on molecular aging pathways and the incidence of age-related multimorbidity and functional decline, is in the right direction to validate MET as an anti-aging drug (Barzilai et al., 2016; Kulkarni et al., 2020).Thus, rigorous investigation of different doses and duration of MET treatment through an improved synergy between preclinical studies and clinical trials would likely validate MET as a drug that improves healthspan in non-diabetics.
Author contributions:LNM wrote the first draft; MK revised the manuscript;AKS edited, revised, and finalized the manuscript text.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by grants from the NationalInstitutes of Health (No.1R01NS106907, to AKS) and the Department of Defense (Nos.W81XWH-17-1-0447 and W81XWH-19-1-0548, to AKS).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ryo Higuchi-Sanabri, University of California Berkeley,USA.
Additional file:Open peer review report 1.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Therapeutic potential of glial cell line-derived neurotrophic factor and cell reprogramming for hippocampal-related neurological disorders
- Influence of Sox protein SUMOylation on neural development and regeneration
- The short form of the SUR1 and its functional implications in the damaged brain
- Oligodendrocyte pathology in fetal alcohol spectrum disorders
- One ring is sufficient to inhibit α-synuclein aggregation
- Neural functions of small heat shock proteins

