Effects of postoperative use of proton pump inhibitors on gastrointestinal bleeding after endoscopic variceal treatment during hospitalization
Yi-Yan Zhang,Le Wang,Xiao-Dong Shao,Yong-Guo Zhang,Shao-Ze Ma,Meng-Yuan Peng,Shi-Xue Xu,Yue Yin,Xiao-Zhong Guo,Xing-Shun Qi
Yi-Yan Zhang,Le Wang,Xiao-Dong Shao,Yong-Guo Zhang,Shao-Ze Ma,Meng-Yuan Peng,Shi-Xue Xu,Yue Yin,Xiao-Zhong Guo,Xing-Shun Qi,Liver Cirrhosis Study Group,Department of Gastroenterology,General Hospital of Northern Theater Command,Shenyang 110840,Liaoning Province,China
Yi-Yan Zhang,Le Wang,Shi-Xue Xu,Yue Yin,Postgraduate College,China Medical University,Shenyang 110122,Liaoning Province,China
Shao-Ze Ma,Postgraduate College,Dalian Medical University,Dalian 116044,Liaoning Province,China
Abstract BACKGROUND Endoscopic variceal treatment(EVT)is recommended as the mainstay choice for the management of high-risk gastroesophageal varices and acute variceal bleeding in liver cirrhosis.Proton pump inhibitors(PPIs)are widely used for various gastric acid-related diseases.However,the effects of PPIs on the development of post-EVT complications,especially gastrointestinal bleeding(GIB),remain controversial.AIMTo evaluate the effects of postoperative use of PPIs on post-EVT complications in patients with liver cirrhosis during hospitalization.METHODS Patients with a diagnosis of liver cirrhosis who were admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command,treated by an attending physician between January 2016 and June 2020 and underwent EVT during their hospitalization were included.Logistic regression analyses were performed to explore the effects of postoperative use of PPIs on the development of post-EVT complications during hospitalization.Odds ratios(ORs)with 95% confidence intervals(CIs)were calculated.RESULTS A total of 143 patients were included.The incidence of post-EVT GIB and other post-EVT complications was 4.90% and 46.85%,respectively.In the overall analyses,postoperative use of PPIs did not significantly reduce the risk of post-EVT GIB(OR = 0.525,95%CI = 0.113-2.438,P= 0.411)or other post-EVT complications(OR = 0.804,95%CI = 0.413-1.565,P= 0.522).In the subgroup analyses according to the enrollment period,type and route of PPIs after the index EVT,use of PPIs before the index EVT,use of vasoactive drugs after the index EVT,indication of EVT(prophylactic and therapeutic),and presence of portal venous system thrombosis,ascites,and hepatocellular carcinoma,the effects of postoperative use of PPIs on the risk of post-EVT GIB or other post-EVT complications remain not statistically significant.CONCLUSION Routine use of PPIs after EVT should not be recommended in patients with liver cirrhosis for the prevention of post-EVT complications during hospitalization.
Key Words:Endoscopic variceal treatment;Gastrointestinal bleeding;Proton pump inhibitors;Complications;Liver cirrhosis;Acute variceal bleeding
lNTRODUCTlON
Acute variceal bleeding(AVB)is a serious complication of liver cirrhosis,indicating the disease progression and development of hepatic decompensation[1,2].Endoscopic variceal treatment(EVT)is recommended as the major choice for the prevention and treatment of AVB[3,4].However,the incidence of post-EVT gastrointestinal bleeding(GIB)ranges from 8% to 25%[5,6],which is mainly due to recurrent varices and post-EVT ulcers[7].In detail,about 4% of patients develop recurrent variceal bleeding after EVT,and 3-25% of patients develop post-EVT ulcer-related GIB[2,8].Notably,the mortality of GIB secondary to post-EVT ulcer is as high as 52%[2].
Considering the benefits of proton pump inhibitors(PPIs)on the prevention of post-EVT GIB[9,10],the American Society for Gastrointestinal Endoscopy recommends the use of PPIs after endoscopic variceal ligation(EVL)to decrease the rate of ligation-induced ulcer[11]and the Chinese Medical Association also recommends the postoperative use of PPIs to improve the hemostasis success and reduce the rates of ulcer and recent post-EVT GIB[12].Indeed,the clinicians often use PPIs after EVT in clinical practice[13].However,the British Society of Gastroenterology states that PPIs are only recommended in the presence of peptic ulcers[14].Additionally,the Baveno VII consensus also states that patients who used PPIs before EVT should discontinue their use immediately after EVT unless they are strictly indicated[3].Recent evidence also suggests that the use of PPIs in patients with liver cirrhosis may increase the risk of hepatic encephalopathy and spontaneous bacterial peritonitis[15].Therefore,whether the routine use of PPIs after EVT is beneficial remains controversial.For this reason,we conducted a retrospective study to evaluate the effects of postoperative use of PPIs on post-EVT GIB and other post-EVT complications in patients with liver cirrhosis during hospitalization.
MATERlALS AND METHODS
Study design
This study was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number[Y(2022)072]and was performed according to the principles of Declaration of Helsinki.The requirement for patients’ informed consent for this study was waived due to its retrospective nature.In this study,we retrospectively reviewed the medical records of 911 patients who were consecutively admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command between January 2016 and June 2020 and treated by an attending physician(XQ)[16-20].We further selected patients who were diagnosed with liver cirrhosis and underwent EVT during their hospitalization.Exclusion criteria were:1)patients who developed GIB or were discharged within 24 h after the index EVT;and 2)patients who started the use of PPIs beyond 24 h after the index EVT.Repeated admissions,malignancies,and other comorbidities were not excluded.
Data extraction
By reviewing electronical medical records,demographic data(i.e.,age and gender),etiologies of liver cirrhosis,laboratory tests(i.e.,white blood cell,hemoglobin,platelet count,total bilirubin,albumin,alanine aminotransferase,serum creatinine,sodium,and international normalized ratio),and other complications of liver cirrhosis[i.e.,ascites,jaundice,hepatic encephalopathy,portal venous system thrombosis(PVST)[17],and hepatocellular carcinoma(HCC)]at admission were collected.Model for end-stage liver disease(MELD)score,Child-Pugh score,and Child-Pugh class at admission were calculated[21].
EVT
All EVT procedures were performed by the same experienced endoscopist(XS)at our department[22,23].EVL and endoscopic cyanoacrylate glue injection(ECGI)were the first-line choices for the management of esophageal and gastric varices,respectively.Endoscopic injection sclerotherapy(EIS)was performed,if EVL was technically difficult,where active massive bleeding impaired visualization or local scar tissue prevented esophageal varices from being aspirated into the cap to achieve ligation.Indication(i.e.,treatment of AVB and primary and secondary prophylaxis of variceal bleeding)and type(i.e.,EVL,ECGI and EIS)of EVT and endoscopic findings[i.e.,grade of esophageal varices(EVs),red sign of EVs,and active bleeding under endoscopy]were reviewed.The use of PPIs before the index EVT and vasoactive drugs(i.e.,octreotide,somatostatin,and terlipressin)after the index EVT were also reviewed.If a patient underwent two or more EVT procedures during the same hospitalization,only the data before the second EVT procedure would be collected.
PPIs after the index EVT
Postoperative PPIs were routinely used in all patients who underwent EVT before January 2018.Since then,this attending physician has systematically reviewed the evidence and questioned the clinical significance of use of PPIs following EVT[10].Thus,postoperative PPIs would be given on demand if a patient was diagnosed with peptic ulcers,esophageal,gastric,and/or duodenal mucosal erosions,or white nipple signs on endoscopy,developed active variceal bleeding during EVT procedures,or complained of acid-related upper gastrointestinal symptoms(i.e.,heartburn and acid regurgitation).Enrollment period,type(i.e.,esomeprazole and pantoprazole),route(i.e.,intravenous and oral),dosage(i.e.,40 mg once daily,40 mg twice daily,and 80 mg twice daily),date of starting and discontinuation,and duration of PPIs after the index EVT were reviewed.These data were extracted until post-EVT GIB,the second EVT procedure,or discharge,whichever came first.
Grouping
Patients were divided into PPIs and non-PPIs groups.The PPIs group was defined as patients who had started on PPIs within 24 h after the index EVT for at least one day before post-EVT GIB,the second EVT procedure,or discharge,whichever came first.The non-PPIs group was defined as patients who had not received PPIs after the index EVT until post-EVT GIB,the second EVT procedure,or discharge,whichever came first(Figure 1).
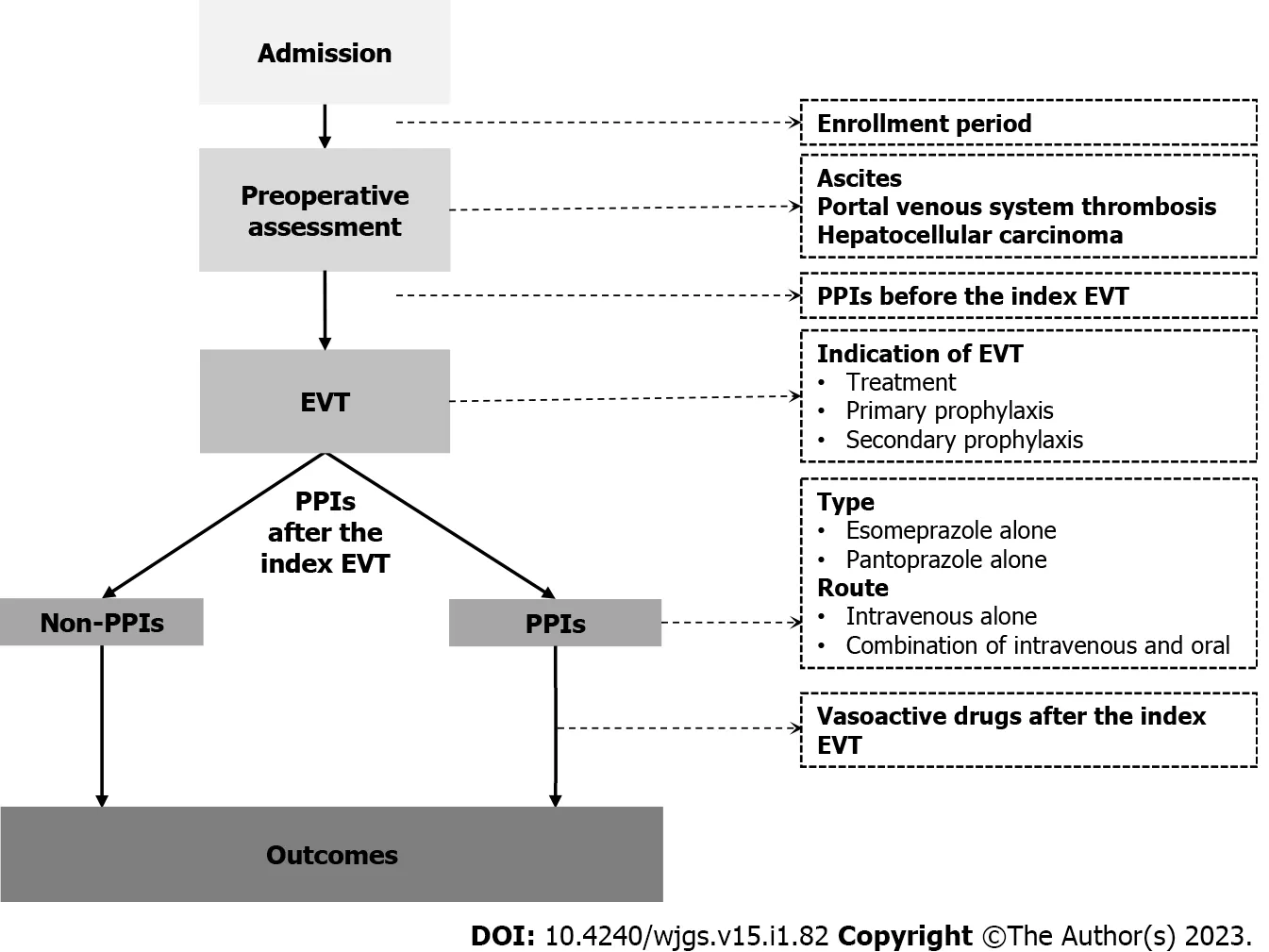
Figure 1 A schematic chart of study design.EVT:Endoscopic variceal treatment;PPIs:Proton pump inhibitors.
Outcomes
The primary outcome was the development of post-EVT GIB during hospitalization.Post-EVT GIB was defined as the presence of hematemesis,and/or melena,and/or hematochezia,and/or firm clinical or laboratory evidence of acute blood loss from the gastrointestinal tract after the index EVT[24].Other post-EVT complications included retrosternal pain/discomfort,nausea/vomiting,heartburn/acid regurgitation,fever,diarrhea,and abdominal pain.
Statistical analyses
All statistical analyses were performed using the IBM SPSS 20.0(IBM Corp,Armonk,NY,USA).Continuous variables were expressed as median(range)and mean ± standard deviation,and categorical variables were expressed as frequency(percentage).The non-parametric Mann-Whitney U test was used to compare continuous variables between PPIs and non-PPIs groups,and the Chi-square test and Fisher's exact test were used to compare categorical variables between the two groups.Logistic regression analyses were performed to explore the impact of postoperative PPIs on post-EVT GIB and other post-EVT complications during hospitalization.Odds ratios(ORs)with 95% confidence intervals(CIs)were calculated.Subgroup analyses were performed according to the enrollment period,type and route of PPIs after the index EVT,use of PPIs before the index EVT,use of vasoactive drugs after the index EVT,indication of EVT,and presence of PVST,ascites,and HCC(Figure 1).A two-sidedP< 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 148 patients with cirrhosis underwent EVT during their hospitalization.Finally,143 patients were included(Figure 2).Of them,83 were in the PPIs group and 60 in the non-PPIs group.The median duration of PPIs administration was 6(1-13)d.The median hospital stay after EVT was 6(2-16)d.Patient characteristics are shown in Table 1.Hepatitis B virus infection alone(36.36%)was the most common etiology of liver cirrhosis followed by alcohol abuse alone(23.08%).The median MELD score and Child-Pugh score were 10.24 and 6.00,respectively.Eighty(55.94%),14(9.79%),6(4.20%),41(28.67%),1(0.70%),and 1(0.70%)patient were treated with EVL alone,ECGI alone,EIS alone,EVL combined with ECGI,EIS combined with ECGI,and EVL combined with ECGI and EIS,respectively(Table 2).
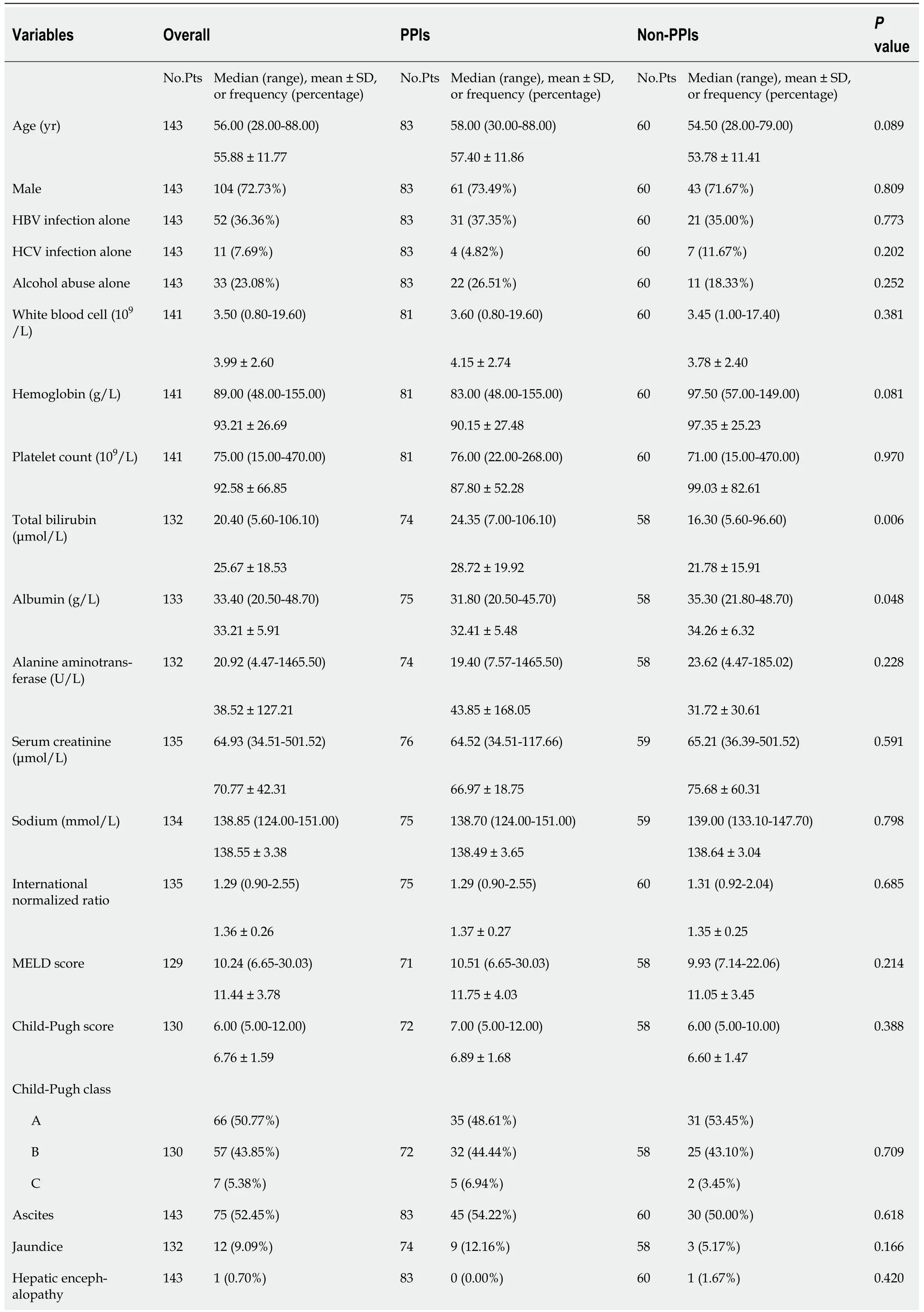
Table 1 Comparison of baseline characteristics between proton pump inhibitors and non-proton pump inhibitors groups
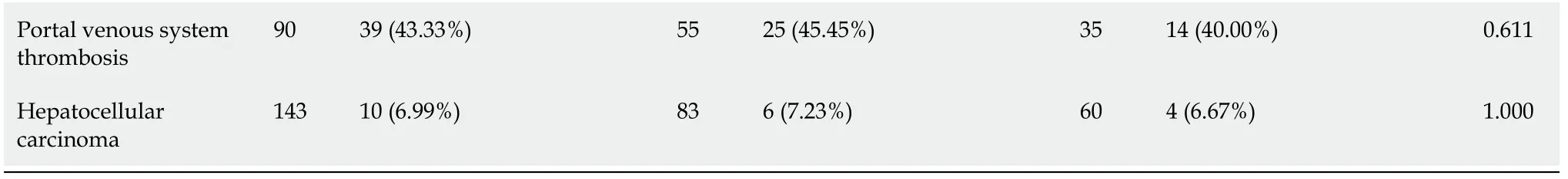
PPIs:Proton pump inhibitors;No.Pts:Numbers of patients;SD:Standard deviation;HBV:Hepatitis B virus;HCV:Hepatitis C virus;MELD:Model for end-stage liver disease.
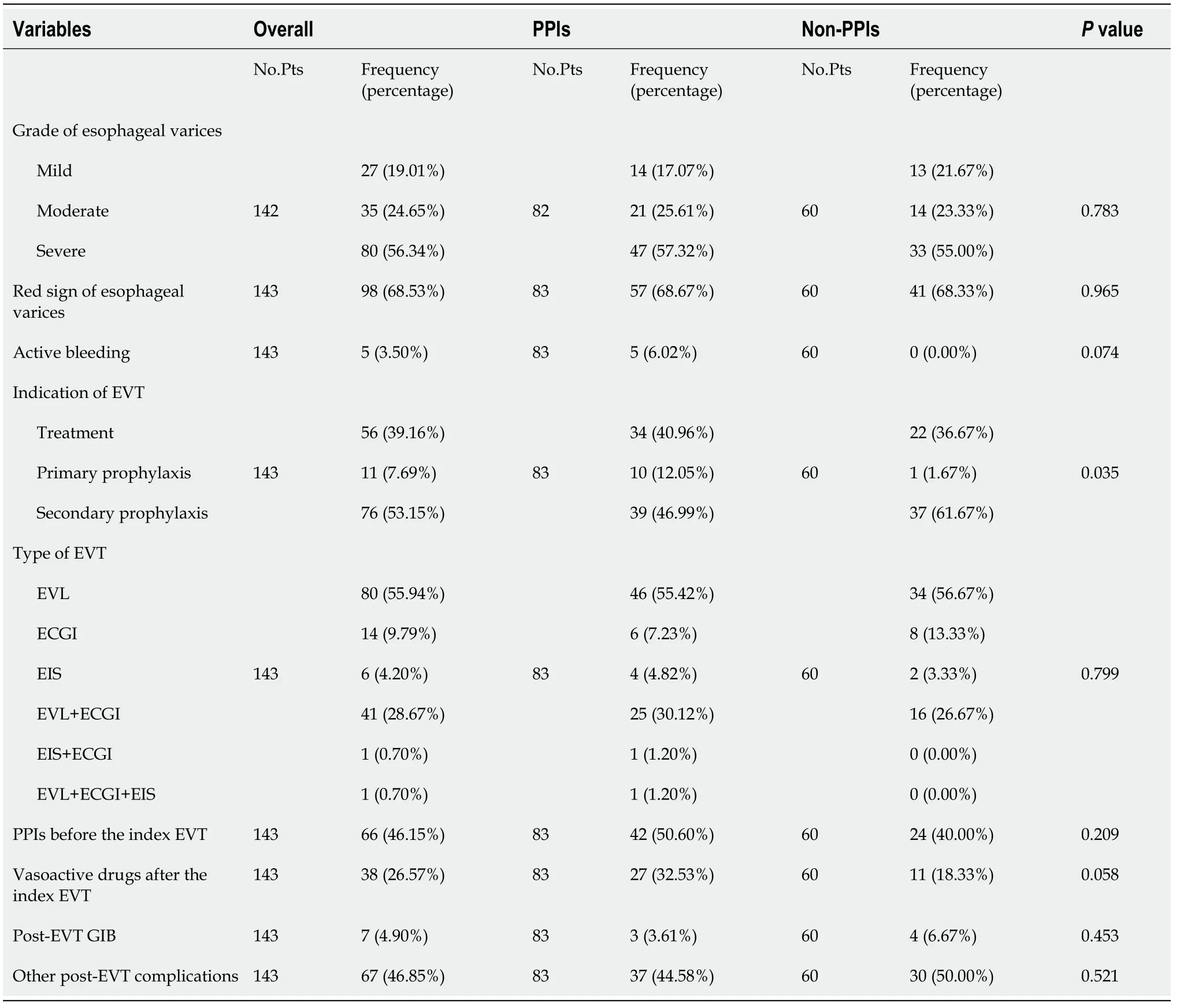
Table 2 Comparison of endoscopic findings,treatment,and outcomes between proton pump inhibitors and non-proton pump inhibitors groups
Overall analyses
Seven(4.90%)patients developed post-EVT GIB,including three in the PPIs group and four in the non-PPIs group.The median interval between the index EVT and post-EVT GIB was 4(2-7)d.Only one of them underwent endoscopy and it was found that the source of post-EVT GIB was a post-EVT ulcer.All of them were administered immediately with intravenous vasoactive drugs for the management of post-EVT GIB and two received blood transfusions.Other post-EVT complications were recorded in 67(46.85%)patients.Logistic regression analyses showed that postoperative use of PPIs was not significantly associated with the risk of post-EVT GIB(OR = 0.525,95%CI = 0.113-2.438,P= 0.411)(Figure 3)or other post-EVT complications(OR = 0.804,95%CI = 0.413-1.565,P= 0.522)(Figure 4).
Subgroup analyses
In all subgroup analyses according to the enrollment period,type and route of PPIs after the index EVT,use of PPIs before the index EVT,use of vasoactive drugs after the index EVT,indication of EVT,and presence of PVST,ascites,and HCC,logistic regression analyses showed that postoperative use of PPIs was not significantly associated with the risk of post-EVT GIB(Figure 3)or other post-EVT complications(Figure 4).
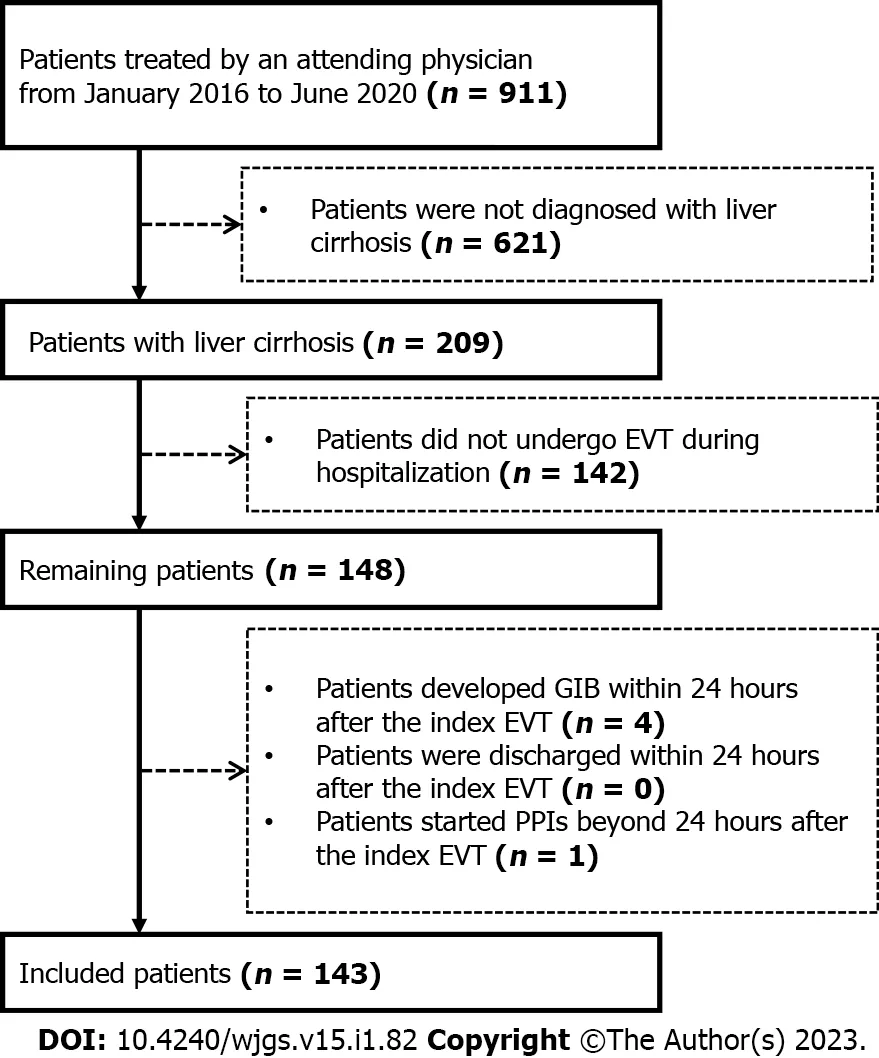
Figure 2 A flow chart of patients’ selection.EVT:Endoscopic variceal treatment;GIB:Gastrointestinal bleeding;PPIs:Proton pump inhibitors.
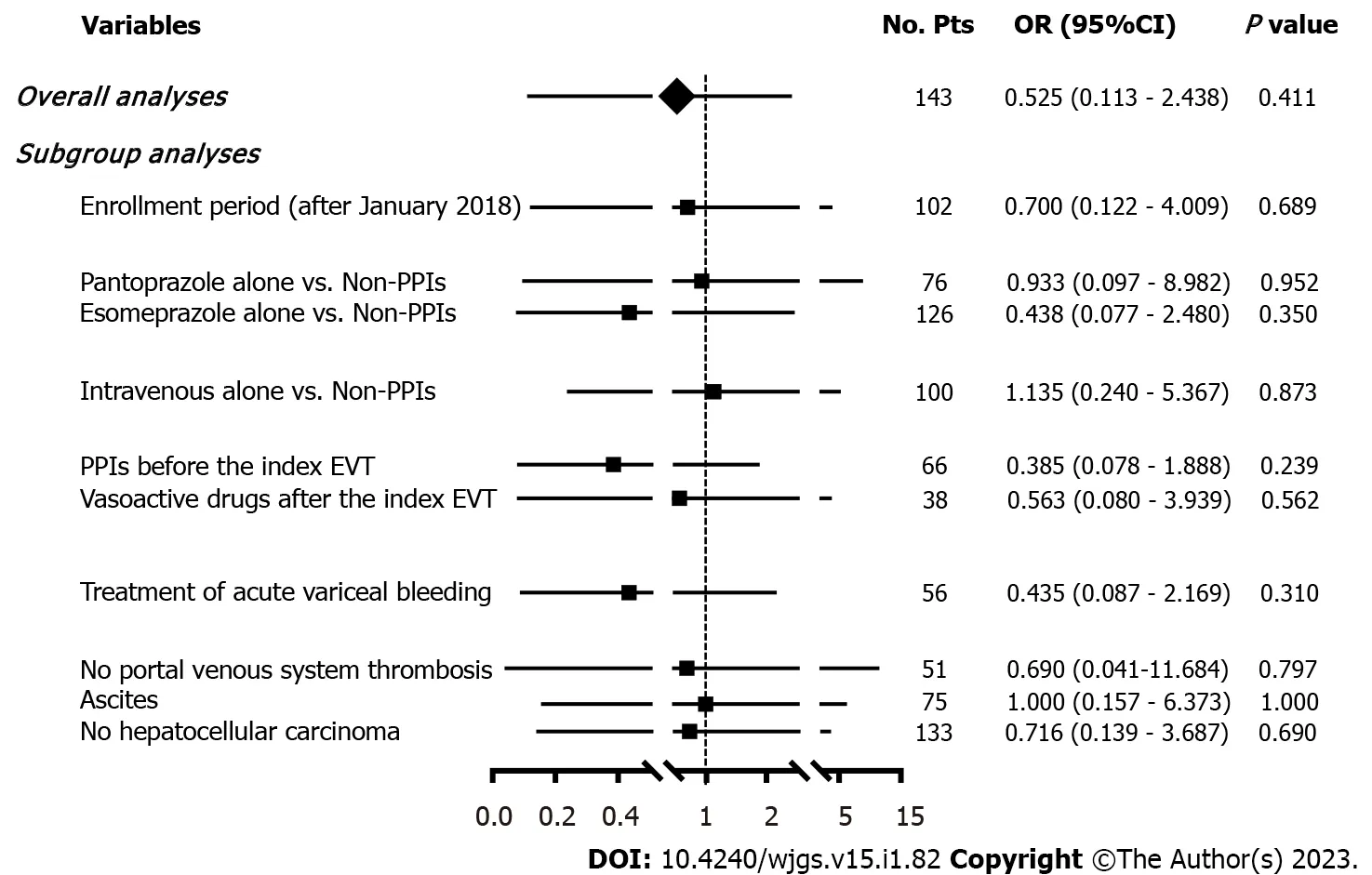
Figure 3 Forest plots showing the effects of postoperative use of proton pump inhibitors on post-EVT GlB during hospitalization.No.Pts:Numbers of patients;OR:Odds ratio;CI:Confidence interval;PPIs:Proton pump inhibitors;EVT:Endoscopic variceal treatment;GIB:Gastrointestinal bleeding.
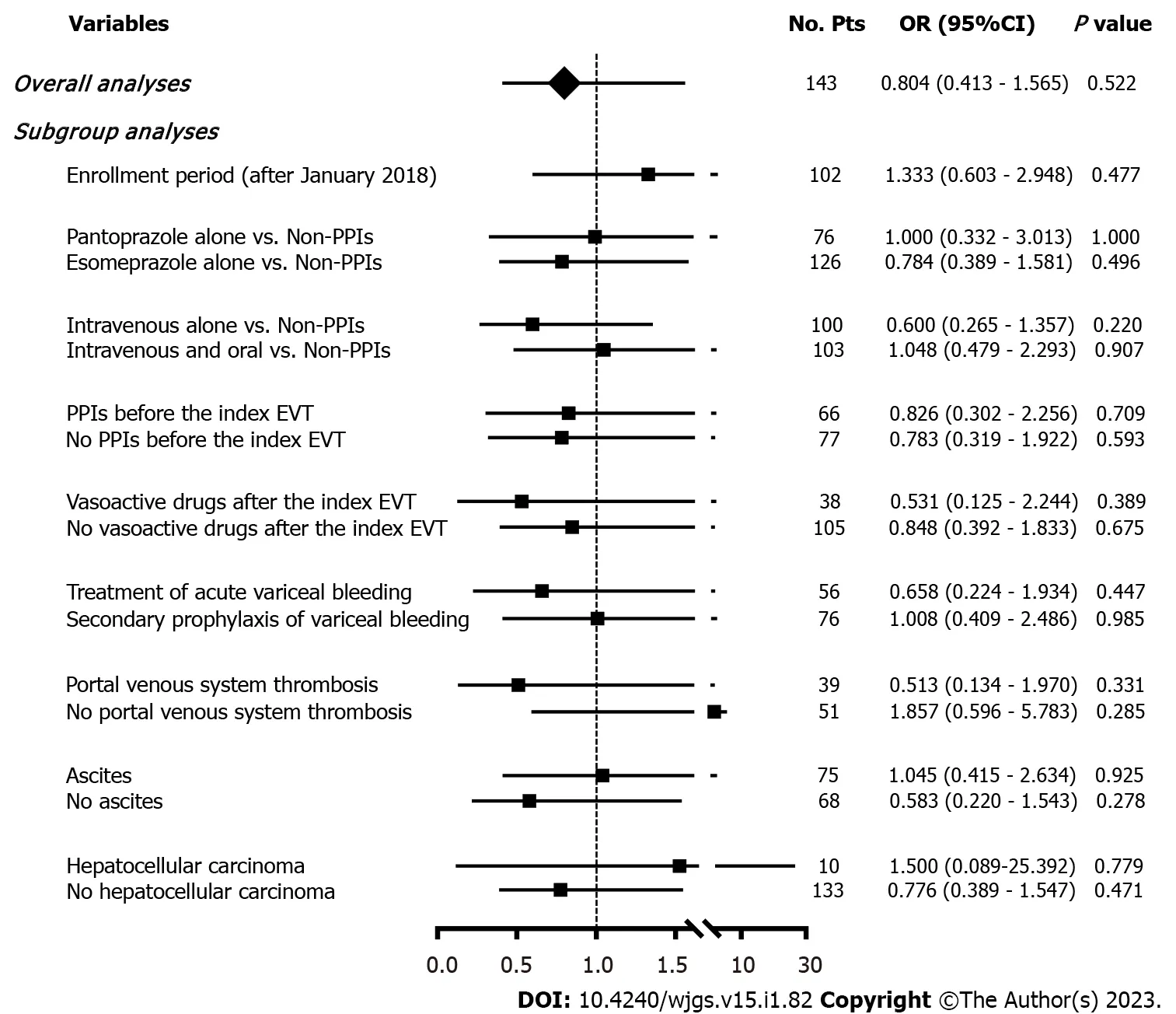
Figure 4 Forest plots showing the effects of postoperative use of proton pump inhibitors on other post-EVT complications during hospitalization.No.Pts:Numbers of patients;OR:Odds ratio;CI:Confidence interval;PPIs:Proton pump inhibitors;EVT:Endoscopic variceal treatment.
DlSCUSSlON
PPIs are one of the most commonly used drugs in the world[25].Increasing evidence suggests that the use of PPIs may reduce the abundance and diversity of gut microbiota,leading to the growth of pathogens and the overgrowth of unhealthy species,and that it may be associated with bone fracture,clostridium difficile infection,spontaneous bacterial peritonitis,and hepatic encephalopathy[25,26].These harms have raised serious concerns about the rational use of PPIs worldwide[27].Therefore,clinicians should carefully consider the postoperative use of PPIs during hospitalization,and assess the optimal effective dosage and duration of PPIs to avoid their related side effects.
Our study found that postoperative use of PPIs had no significant effect on post-EVT GIB and other post-EVT complications.Our study has several advantages in terms of study design.First,all patients were diagnosed and treated by the same attending physician and all EVT procedures were also performed by the same endoscopist,which avoids heterogeneity in the management of patients.Second,patients who underwent prophylactic and therapeutic EVT procedures were both included.Third,subgroup analyses were comprehensively performed according to the enrollment period,type and route of PPIs after the index EVT,use of PPIs before the index EVT,use of vasoactive drugs after the index EVT,indication of EVT(prophylactic and therapeutic EVT),and presence of PVST,ascites,and HCC,which minimizes the impact of confounding factors on statistical results.Fourth,all included patients had been evaluated for at least 24 h since the index EVT,which potentially rules out the effect of technical failure on patients’ outcomes.
Post-EVT ulcer,which is one of the main causes of post-EVT GIB,is primarily due to early slippage of rubber bands,sclerosant-induced inflammatory necrosis,and tissue glue-induced caseous necrosis[7,28-31].It has been traditionally believed that the presence of gastric acid delays ulcer healing[32].Esophageal motility may be temporarily impaired due to nerve plexus injury after EVT,which delays gastric acid clearance and aggravates the progression of ulcers[33,34].PPIs are potent acid inhibitors widely used for various acid-related diseases and may promote early healing of post-EVT ulcers by reducing gastric acid secretion,thereby probably decreasing the risk of post-EVT GIB[26,32,35].In contrast,our study did not demonstrate the benefits of postoperative PPIs in reducing the development of post-EVT GIB.There are some explanations for this unexpected phenomenon.First,post-EVT ulcers are more prone to develop bleeding primarily due to persistent portal hypertension,but not gastric acid[4,31].Second,the use of PPIs can only reduce the size of ulcers,but not the number of ulcers[36].Notably,the size of ulcers is not associated with the risk of bleeding[36].Third,we only observed the impact of short-term use of PPIs on the development of post-EVT GIB during hospitalization.However,post-EVT ulcer healing often requires a duration of about 2 wk[37,38].
Our previous meta-analysis showed a significant benefit of PPIs on post-EVT GIB in patients who underwent prophylactic EVL,but not therapeutic EVT[10].However,the present study could not confirm the protective effect of postoperative use of PPIs on GIB after prophylactic EVT,because none of the patients who underwent EVT for primary or secondary prophylaxis of variceal bleeding developed post-EVT GIB.Nevertheless,it has been proposed that post-EVL ulcers are usually shallower with only superficial mucosal damage,which may heal more easily with the use of PPIs[37].Patients who need EVT for the treatment of AVB often have a white nipple,red nipple,or mucosal erosion on endoscopy.Undoubtedly,their conditions are more severe,where the anti-acid effect of PPIs may be insufficient for the improvement of ulcer healing[4].
Except for post-EVT GIB,EVT can also cause other procedure-related complications,which are mild and reversible[3,7].We did not find any significant effect of PPIs on the development of other post-EVT complications.This can be explained by the fact that only a fraction of post-EVT complications,such as acid regurgitation and heartburn,are related to gastric acid[39].By comparison,retrosternal discomfort/pain,nausea,and vomiting are mostly mechanical injuries caused by EVT,and fever may be secondary to bacterial infection[40,41].Garget al[28]also achieved similar findings,but Loet al[42]showed fewer complications in patients receiving PPIs.Such a discrepancy might be related to the type of complications evaluated,endoscopic techniques,and patients’ conditions.
Our study has some limitations.First,the total number of the patients included was small in this study.Second,there were a few cases of post-EVT GIB,which made our statistical analyses underpowered and increased the possibility of type II errors(i.e.,false-negative findings).Third,only one patient who developed post-EVT GIB underwent second-look endoscopy,because all of the six patients who developed post-EVT GIB were successfully treated with pharmacotherapy.Fourth,none died of post-EVT GIB or other causes during hospitalization,compromising further analyses regarding the impact of PPIs on death.Fifth,follow-up data were lacking to assess the 6-wk and long-term mortality.
CONCLUSlON
Our study suggested that postoperative use of PPIs could not reduce the development of post-EVT GIB and other post-EVT complications during hospitalization.Therefore,PPIs after EVT should not be routinely used during hospitalization,and their indications should be carefully evaluated.
ARTlCLE HlGHLlGHTS
Research background
Endoscopic variceal treatment(EVT)is frequently used in cirrhosis with high-risk gastroesophageal varices and acute variceal bleeding.However,it is often associated with a high risk of post-EVT complications,especially postoperative gastrointestinal bleeding(GIB).
Research motivation
The role of proton pump inhibitors(PPIs)after EVT remains controversial.
Research objectives
To evaluate the impact of postoperative use of PPIs on post-EVT GIB and other post-EVT complications in patients with liver cirrhosis during hospitalization.
Research methods
We retrospectively reviewed 911 patients who were consecutively admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command between January 2016 and June 2020 and treated by an attending physician.Logistic regression analyses were performed to explore the impact of postoperative PPIs on post-EVT GIB and other post-EVT complications during hospitalization.
Research results
A total of 143 patients were included.The incidence of post-EVT GIB and other post-EVT complications was 4.90% and 46.85%,respectively.In either overall or subgroup analyses,postoperative use of PPIs did not significantly reduce the risk of post-EVT GIB or other post-EVT complications.
Research conclusions
Postoperative use of PPIs was not beneficial for reducing the development of post-EVT GIB and other post-EVT complications during hospitalization.
Research perspectives
PPIs after EVT should not be routinely used during hospitalization,and their indications should be carefully evaluated.Prospective studies are required to further validate the conclusions of this study.
ACKNOWLEDGEMENTS
We are indebted to our study team,including Wen-Chun Bao,Fei-Fei Hou,Ze-Qi Guo,Jing-Qiao Zhang,Xin-Miao Zhou,Miao-Miao Li,Yang An,Rui-Rui Feng,Cen Hong,Yang-Lan He,Hai-Juan Yao,and Le Wang,for establishing and updating the database which prospectively recorded the patients treated by Dr.Xing-Shun Qi.
FOOTNOTES
Author contributions:Qi XS contributed to conceptualization;Zhang YY,Wang L,Shao XD,Zhang YG,Ma SZ,Peng MY,Xu SX,Yin Y,Guo XZ,and Qi XS contributed to methodology;Zhang YY and Qi XS contributed to formal analysis;Zhang YY,Wang L,Shao XD,Zhang YG,Ma SZ,Peng MY,Xu SX,Yin Y,Guo XZ,and Qi XS contributed to data curation;Zhang YY and Qi XS contributed to writing original draft;Zhang YY,Wang L,Shao XD,Zhang YG,Ma SZ,Peng MY,Xu SX,Yin Y,Guo XZ,and Qi XS contributed to writing review and editing;Guo XZ and Qi XS contributed to supervision;Qi XS contributed to project administration;all authors have read and approved the final manuscript.
lnstitutional review board statement:This study has been approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number[Y(2022)072]and was performed according to the Declaration of Helsinki.
lnformed consent statement:The requirement for patients' informed consent for this study was waived due to its retrospective nature.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:The dataset of the current study is available from the corresponding author on reasonable request.
STROBE statement:The authors have read the STROBE Statement-checklist of items,and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yi-Yan Zhang 0000-0003-4550-8724;Le Wang 0000-0002-1475-0334;Xiao-Dong Shao 0000-0002-7693-2969;Yong-Guo Zhang 0000-0001-9330-1651;Shao-Ze Ma 0000-0002-8298-9435;Meng-Yuan Peng 0000-0003-0391-7300;Shi-Xue Xu 0000-0002-9928-6074;Yue Yin 0000-0003-3279-4602;Xiao-Zhong Guo 0000-0002-6397-0501;Xing-Shun Qi 0000-0002-9448-6739.
S-Editor:Liu GL
L-Editor:Ma JY - MedE A
P-Editor:Liu GL
 World Journal of Gastrointestinal Surgery2023年1期
World Journal of Gastrointestinal Surgery2023年1期
- World Journal of Gastrointestinal Surgery的其它文章
- Hereditary polyposis syndromes remain a challenging disease entity:Old dilemmas and new insights
- Application of ablative therapy for intrahepatic recurrent hepatocellular carcinoma following hepatectomy
- Postoperative adjuvant therapy for hepatocellular carcinoma with microvascular invasion
- Prognostic effect of excessive chemotherapy cycles for stage ll and lll gastric cancer patients after D2 + gastrectomy
- Development and validation of a novel nomogram for predicting overall survival in gastric cancer based on inflammatory markers
- New perspectives on robotic pancreaticoduodenectomy:An analysis of the National Cancer Database
