Role of the nerve growth factor precursor-neurotrophin receptor p75 and sortilin pathway on apoptosis in the brain of patients with intracerebral hemorrhage*☆
Gang Bao, Qi Li, Yuliang Han, Ning Wang, Shiwen Guo, Jinning Song, Baixiang He,Kai Wang
1Department of Neurosurgery, the First Affiliated Hospital of Medical College of Xi'an Jiaotong University, Xi'an 710061, Shaanxi Province,China
2Department of Neurology, the 305 Hospital of PLA, Beijing 100017, China
lNTRODUCTlON
Intracerebral hemorrhage may induce direct effects at and around the site of the lesion resulting in acute injury to brain tissues[1-3].
However, other cellular processes such as apoptosis, may also induce other secondary effects following intracerebral hemorrhage,with the capacity to exacerbate the pathogenetic pathways[4]. The molecular mechanism underlying cellular apoptosis in the tissues surrounding intracerebral hematomas following hemorrhage remains unclear.
The neurotrophin receptor p75 (p75NTR),locating on the transmembrane domain near the membrane adjacent region, is named the Chopper short-peptide region[5]. Being palmitoylated, p75NTRmay induce death in and non-neural cells[6-7]. The physiological activities of p75NTRcan be broadly considered one of two kinds, one where p75NTRcollaborates with the Trk receptor to increase or decrease the effects of the neurotrophin receptor on the Trk receptor,and the other is the close relationship between p75NTRand apoptosis. In particular,nerve growth factor precursor (proNGF)-induced p75NTR-mediated expression is one means of promoting cellular apoptosis[8].
ProNGF mediates cellular apoptosis through the formation of a ligand-receptor complex with p75NTRand sortilin. Moreover, sortilin acts as an assistant receptor and molecular switch for controlling the proNGF-induced p75NTR- mediated signals[1-2].
To date, few reports have investigated the role of the p75NTR-mediated apoptosis pathway in intracerebral hemorrhage. We hypothesize that the proNGF-p75NTR-sortilin heterotrimer-mediated apoptotic pathway participates in cellular apoptosis following hemorrhage. The aim of this study was to determine whether there are any differences in the amount of apoptosis, expression of proNGF, p75NTRor sortilin in the brain regions surrounding the site of hematoma following the intracerebral hemorrhage, and to investigate the relationship between expression of these key genes and cellular apoptosis.
RESULTS
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)assays revealed an increase in the number of apoptotic cells in cerebral tissue from patients following intracerebral hemorrhage
Samples from the hemorrhagic group demonstrated areas of edematous swelling, particularly of the neurons and glial cells, around the site of the hematoma. A large number of vacuoles were also detected and there was evidence of neuronal membrane breakage and poor staining of the cytoplasm. The cells presented with an irregular morphology coupled with the presences of cellular necrosis. Some cells had disappeared leaving only a deeply stained and shrinking nucleus. The capillaries were frequently enlarged and congested, with swelling at the vessel walls. Statistical analysis revealed that a significant number of glial cells,which were characterized with yellow stained nucleus,were found in the area surrounding the lesion.
Conversely, only few neurons were observed in this region and found to be apoptotic. However, in the control group, the degree of tissue edema was considerably less and there was no obvious swelling of the neurons or glial cells. Necrotic neurons were occasionally encountered within the visual field. Glial cells and microvascular spaces were not evident within the relatively dense matrix. In addition, only a small number of cells with a yellow nucleus were present, coupled with a lower number of apoptotic and fewer neurons. Approximately one third of the neurons were TUNEL-positive, which was lower than that in the hemorrhagic group (P < 0.05;Figure 1, Table 1, supplementary Figures 1-4 online).

Figure 1 Apoptotic cells detected by terminal deoxynucleotidyl transferase dUTP nick end labeling assay.
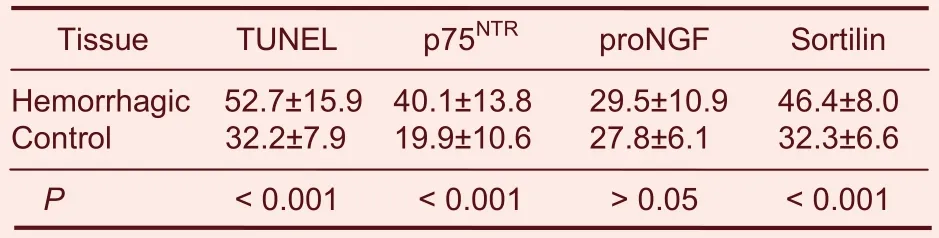
Table 1 Comparisons of the number of terminal deoxynucleotidyl transferase dUTP nick end labeled(TUNEL), p75 neurotrophin receptor (p75NTR), nerve growth factor precursor (proNGF) and sortilin-positive cells in hemorrhagic and normal (control) brain tissue(mean ± SD, n = 31, %)
lmmunohistochemistry revealed increased p75NTR and sortilin expression in the hemorrhagic group
As outlined in Figures 2-4, the expression of p75NTR,proNGF and sortilin in neurons was higher than that in neural glial cells. The expression of p75NTR, proNGF and sortilin was evident in both the hemorrhagic and control groups. However, the number of p75NTR- and sortilin- positive cells was significantly higher in the hemorrhagic group than in the control group (P < 0.001),while there was no statistical significance difference in proNGF expression between the two groups (Table 1).
A positive correlation was detected between the TUNEL and p75NTR-positive cells (r = 0.705, P < 0.001;Figure 5).
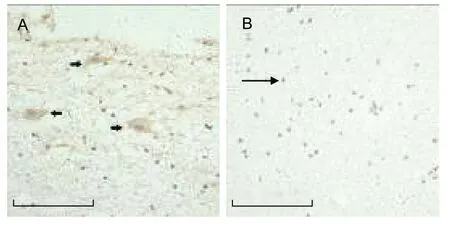
Figure 2 Analysis of p75 neurotrophin receptor (p75NTR)staining.
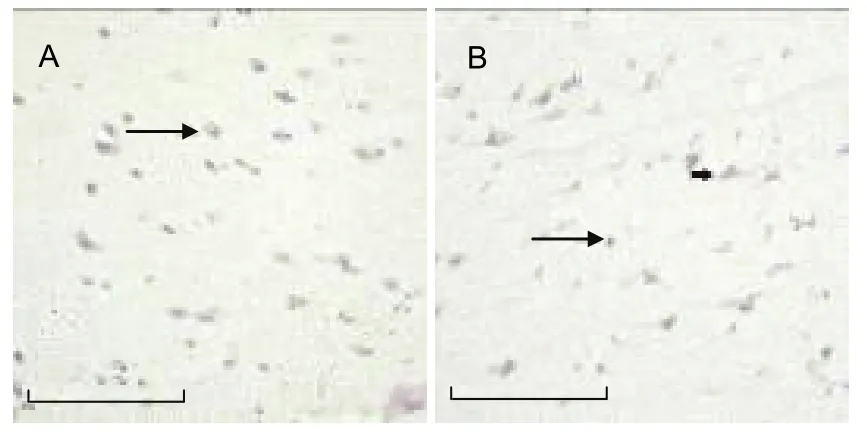
Figure 3 Analysis of nerve growth factor precursor(proNGF) expression.
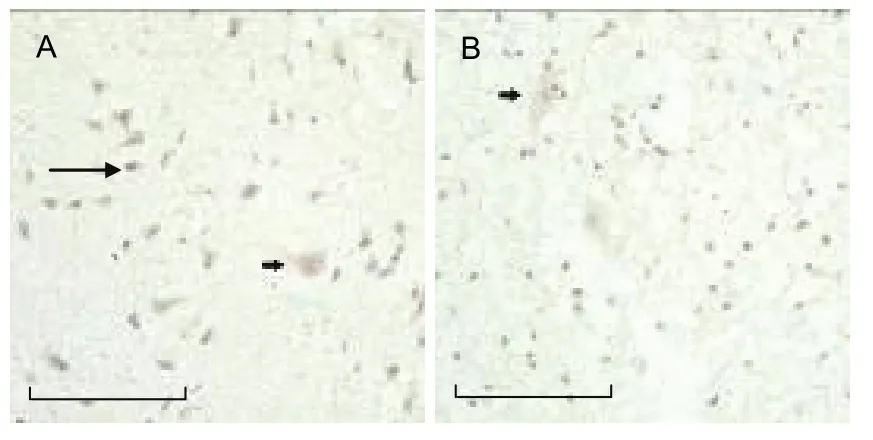
Figure 4 Analysis of sortilin expression.
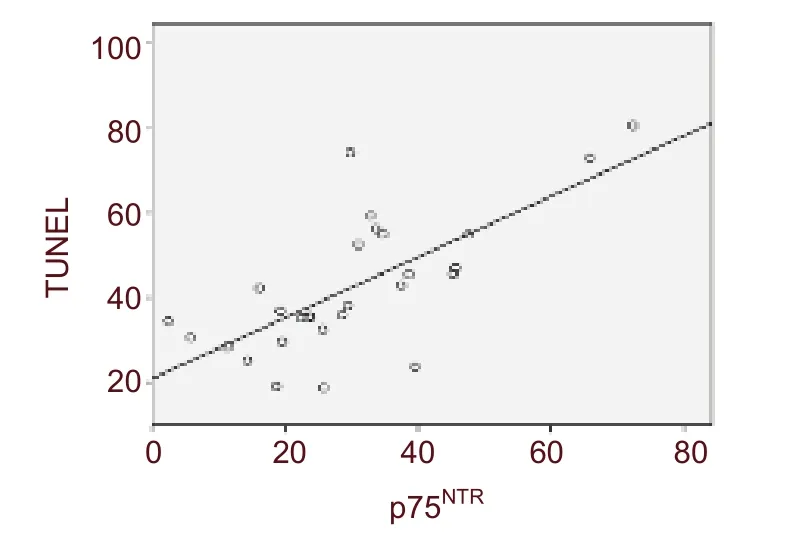
Figure 5 Analysis of the correlation between p75 neurotrophin receptor (p75NTR) and terminal deoxynucleotidyl transferase dUTP nick end labeling(TUNEL) expression. Results revealed that there was a high degree of correlation between p75NTR and TUNEL expression (r = 0.705, P < 0.001).
DlSCUSSlON
Secondary injury through molecular pathways such as apoptosis and inflammation are an important cause of brain damage in patients with intracerebral hemorrhage,as the direct effects of the hematoma[7-9]. In this study,apoptotic cells, which mainly consisted of neurons and neural glial cells were detected in all the samples collected from the tissues surrounding of the site of a intracerebral hematoma. Previous work has shown that apoptosis occurs in perihematomal brain regions following intracerebral hemorrhage. This may correlate with the decreased local cerebral blood flow and energy metabolism, inflammatory reactions in perihematomal tissues, and the release of thrombin[10-13]. Results from the present study suggest that apoptotic cells, neurons and glial cells in particular, were observed in the perihematomal region of all brain samples analyzed. The finding showed that TUNEL-positive cells were also observed in the control group, suggests that distance from the lesion might induce various effects on intracerebral hemorrhage.
Paired t-test analysis revealed that the apoptotic ratio significantly increased in the hemorrhagic group compared with the control group, suggesting that cellular apoptosis is an important factor contributing to secondary cerebral injury following intracerebral hemorrhage. This experiment showed that there were significant differences in p75NTRexpression between the hemorrhagic group and the control group. There was also a positive correlation between p75NTRexpression and apoptosis. Thus we speculated p75NTR-dependent signaling pathways play an important role in the onset of apoptosis following intracerebral hemorrhage. It was interesting to note that p75NTRexpression in the hemorrhagic samples varied with respect to the degree of cellular apoptosis observed. This difference was only observed after detailed experimental analysis. However,it is still unclear whether an interaction between proNGF and TrkA is present, and how the microenvironment changed under the hemorrhage-induced apoptotic status.
In addition, the proNGF-receptor interaction-mediated downstream signal transduction pathway and apoptotic mechanisms are yet to be elucidated.
Nerve growth factor increases neuronal survival, and is a polypeptide that promotes neuronal survival and growth.
However, proNGF does not exhibit any biological activity[14]. According to previous studies, proNGF induces cellular apoptosis[15-19]. High-affinity proNGF combined with the target cell membrane p75NTRreceptor may induce p75NTR-mediated cell apoptosis. Moreover, high-affinity mNGF combined with the TrkA receptor increases neuronal survival and axonal growth[4,16,20-22]. The findings from the current study demonstrated no significant difference in proNGF protein expression between the hemorrhage and control groups. However, increased proNGF expression has been shown in trauma models and other rat models of disease[18,23]. In this study,proNGF protein expression was unaltered, suggesting that proNGF did not have any effects on cellular apoptosis following intracerebral hemorrhage. Work by another group found that proNGF expression in the hemorrhage group enhanced p75NTRexpression, as well as the number of apoptotic cells, indicating that proNGF-induced cellular apoptosis or cell survival correlates with the cleavage of proNGF[20]. Furthermore,cellular apoptosis following intracerebral hemorrhage promoted binding between proNGF and p75NTRdue to increased p75NTRexpression at the surface of the cell membrane[5]. However, proNGF could not be detected by immunohistochemistry because of internalization. It is likely that proNGF cleavage plays a key role in regulating and controlling the biological actions of neurotrophin and secondary cerebral injury.
Sortilin acts as a necessary factor for the proNGF induction of p75NTR-mediated cellular death.
Sortilin-specific antibodies have been shown to block cell death[18,24-27]. Sortilin, p75NTR, and proNGF form a trimer that induces biological action. Sortilin acts as an assistant receptor and molecular switch to control signals prior to p75NTR-mediated proNGF-induced apoptosis[28-30]. Although proNGF expression was not altered following intracerebral hemorrhage, sortilin expression increased and was positively correlated with the number of apoptotic cells. In the present study, when sortilin expression increased, proNGF expression increased, the proNGF-sortilin-p75NTRtrimer increased, and the neurotrophic effect of mNGF decreased. In contrast, increased sortilin expression promoted combination with proNGF, but did not influence proNGF expression.
In conclusion, proNGF expression was not altered in the perihematomal brain region following intracerebral hemorrhage. Sortilin expression increased and was positively correlated with apoptosis. Sortilin acted as an assistant receptor and molecular switch for p75NTR-mediated apoptosis.
MATERlALS AND METHODS
Design
A controlled observation pertaining to molecular biology.
Time and setting
These experiments were performed at the Laboratory of Histology and Embryology, Xi’an Jiaotong University, China from December 2009 to June 2010.
Materials
All the brain samples were collected from intracerebral hemorrhagic patients who were treated at the Department of Neurosurgery, the First Hospital of Xi’an Jiaotong University, China, from December 2009 to June 2010. Thirty-one patients were involved and all met the operation indications, which were as follows: more than 30 mL of supratentorial hemorrhage and more than 10 mL of cerebral hemorrhage. All harvested brain tissue was removed during the surgical approach. Other than the operation the patients incurred no additional insults.
Informed consents was obtained from the patients themselves or authorized relatives. This experiment was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, China.
Methods
Grouping
A total of 31 patients with basal ganglia hemorrhage whose hematoma volume reached surgical standards were selected. Craniotomy or micro-trauma ossicle fenestration procedure were performed to cut the endocranium and gyrus temporalis medius. A small amount of the tissues (approximately 0.5 cm)surrounding hematoma was collected for inclusion within the intracerebral hemorrhagic group. Cerebral tissues from the gyrus temporalis medius, distant from the hematoma, were collected as the control specimens.
Cellular apoptosis of the cerebral tissues as determined by TUNEL
The brain samples obtained during surgery were formalin-fixed and cut into serial sections following paraffin embedding. The 7 μmol/L-thick sections were fixed to glass slides and placed in 60°C oven for 48 hours. The slides were then dewaxed and rehydrated.
Peroxidase blocking solution was dropped onto each of samples after high-pressure fixed antigen, and then the samples were incubated for 10 minutes at 37°C. Normal goat serum was then added to the samples and incubated for 3 minutes at 37°C. The sections were then fixed in 40 g/L paraformaldehyde for 15 minutes (room temperature), followed by 20 μg/mL proteinase K solution(Promega, Madison, WI, USA) and the proteins were extracted after 15 minutes of hydrolysis at room temperature, followed by the addition of 100 μL TdT enzyme buffer (Promega) for 10 minutes at room temperature. The reagents reacted for 30 minutes in a humidified chamber stored at room temperature after the addition of 100 μL horseradish peroxidase-labeled streptavidin (Promega). Similarly, 100 μL of freshly prepared diaminobenzidine (Promega) solution was dropped directly on the tissue sections for 6 minutes. The slides were then counterstained with hematoxylin(Biosynthesis Biotechnology, Beijing, China) after anhydration. The apoptotic cells were detected using an optical microscope (Leica, Wetzlar, Germany)[11].
Immunohistochemistry was used to determine p75NTR, proNGF and sortilin expression
The sections were dewaxed, hydrated, and incubated in a 3% hydrogen peroxide solution to remove endogenous peroxide. Antigen retrieval was performed by heating the sections, which were then blocked with goat serum for 20 minutes, and subsequently stained according to previously described methods. The sections were incubated overnight at 4°C in a rabbit anti-human p75NTRpolyclonal antibody (1: 1 000; Biosynthesis Biotechnology), rabbit anti-human sortilin polyclonal antibody (1: 700; Abcam, Cambridge, UK) and rabbit anti-human proNGF polyclonal antibody (1: 250;Chemicon, Temecula, CA, USA). Secondary detection consisted of biotin-labeled goat anti-rabbit IgG(Biosynthesis Biotechnology) for 20 minutes at 37°C, and alkaline phosphatase-labeled streptavidin at 37°C for 30 minutes. The sections were then stained with DAB(Biosynthesis Biotechnology), counterstained with hematoxylin (Biosynthesis Biotechnology), dried and finally mounted. The p75NTR, proNGF and sortilin expressions were detected under an optical microscope(Leica)[21].
Statistical analysis
Statistical analysiswas performed with SPSS 15.0 software (SPSS Inc, Chicago, IL, USA). Since the cerebral tissue distant from the hematoma in each patient was used as his/her own control, continuous variables (presented as mean ± SD) were compared using a paired Student’s t-test. A probability value of < 0.05 was considered statistically significant.
Author contributions:Gang Bao proposed and designed the study, wrote the manuscript and obtained the funding. Qi Li was responsible for the data analysis and manuscript preparation.Yuliang Han provided and integrated the data. Ning Wang was responsible for the data and statistical analysis. Shiwen Guo validated the paper and instructed the experiments. Jinning Song offered the technical and/or data support. Baixiang He and Kai Wang provided the experimental information.
Conflicts of interest:None declared.
Funding:This study was financially supported by the Science and Technology Research and Development Program of Shaanxi Province, No. 2007K15-01.
Ethical approval:This experiment was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, China.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 6, No. 22, 2011 item after selecting the “NRR Current Issue” button on the page.
[1]Illanes S, Zhou W, Heiland S, et al. Kinetics of hematoma expansion in murine warfarin-associated intracerebral hemorrhage. Brain Res. 2010;1320:135-142.
[2]Steiner T, Bosel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke. 2010;41(2):402-409.
[3]McGuire AJ, Raikou M, Whittle I, et al. Long-term mortality,morbidity and hospital care following intracerebral hemorrhage:an 11-year cohort study. Cerebrovasc Dis. 2007;23(2-3):221-228.
[4]Han N, Ding SJ, Wu T, et al. Correlation of free radical level and apoptosis after intracerebral hemorrhage in rats. Neurosci Bull.2008;24(6):351-358.
[5]Nykjaer A, Willnow TE, Petersen CM. p75NTR--live or let die. Curr Opin Neurobiol. 2005;15(1):49-57.
[6]Angelo MF, Aviles-Reyes RX, Villarreal A, et al. p75(NTR)expression is induced in isolated neurons of the penumbra after ischemia by cortical devascularization. J Neurosci Res. 2009;87(8):1892-903.
[7]Moussouttas M, Malhotra R, Fernandez L, et al. Role of antiplatelet agents in hematoma expansion during the acute period of intracerebral hemorrhage. Neurocrit Care. 2010;12(1):24-29.
[8]Sheth KN, Cushing TA, Wendell L, et al. Comparison of hematoma shape and volume estimates in warfarin versus non-warfarin-related intracerebral hemorrhage. Neurocrit Care.2010;12(1):30-34.
[9]Zhang XQ, Zhang ZM, Yin XL, et al. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chin Med J (Engl).2010;123(10):1246-1250.
[10]Ramos-Cabrer P, Campos F, Sobrino T, et al. Targeting the ischemic penumbra. Stroke. 2011;42(1 Suppl):S7-11.
[11]Qureshi AI, Suri MF, Ostrow PT, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52(5):1041-1048.
[12]Thiex R, Tsirka SE. Brain edema after intracerebral hemorrhage:mechanisms, treatment options, management strategies, and operative indications. Neurosurg Focus. 2007;22(5):E6.
[13]Jung KH, Chu K, Lee ST, et al. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther. 2007;322(3):1051-1058.
[14]Turner BJ, Cheah IK, Macfarlane KJ, et al. Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem. 2003;87(3):752-763.
[15]Al-Shawi R, Hafner A, Olson J, et al. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur J Neurosci. 2008;27(8):2103-2114.
[16]Lee R, Kermani P, Teng KK, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945-1948.
[17]Hempstead BL. Commentary: Regulating proNGF action: multiple targets for therapeutic intervention. Neurotox Res. 2009;16(3):255-260.
[18]Jansen P, Giehl K, Nyengaard JR, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10(11):1449-1457.
[19]Wang YJ, Valadares D, Sun Y, et al. Effects of proNGF on neuronal viability, neurite growth and amyloid-beta metabolism.Neurotox Res. 2010;17(3):257-267.
[20]Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave.Neuron. 2002;33(1):9-12.
[21]Stoica G, Lungu G, Kim HT, et al. Up-regulation of pro-nerve growth factor, neurotrophin receptor p75, and sortilin is associated with retrovirus-induced spongiform encephalomyelopathy. Brain Res. 2008;1208:204-216.
[22]Teng KK, Felice S, Kim T, et al. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70(5):350-359.
[23]Al-Gayyar MM, Matragoon S, Pillai BA, et al. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected]. Diabetologia.2011;54(3):669-680.
[24]Nakamura K, Namekata K, Harada C, et al. Intracellular sortilin expression pattern regulates proNGF-induced naturally occurring cell death during development. Cell Death Differ. 2007;14(8):1552-1554.
[25]Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843-848.
[26]Terry AV, Kutiyanawalla A, Pillai A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiol Behav. 2011;102(2):149-157.
[27]Tauris J, Gustafsen C, Christensen EI, et al. Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons. Eur J Neurosci. 2011;33(4):622-631.
[28]Quistgaard EM, Madsen P, Groftehauge MK, et al. Ligands bind to Sortilin in the tunnel of a ten-bladed beta-propeller domain. Nat Struct Mol Biol. 2009;16(1):96-98.
[29]Paiardini A, Caputo V. Insights into the interaction of sortilin with proneurotrophins: a computational approach. Neuropeptides.2008;42(2):205-214.
[30]Chen LW, Yung KK, Chan YS, et al. The proNGF-p75NTR-sortilin signalling complex as new target for the therapeutic treatment of Parkinson's disease. CNS Neurol Disord Drug Targets. 2008;7(6):512-523.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Meta-analysis of transcranial magnetic stimulation to treat post-stroke dysfunction○
- Correlation of E-selectin gene polymorphisms with risk of ischemic stroke A meta-analysis☆
- Penehyclidine hydrochloride attenuates cerebral vasospasm after subarachnoid hemorrhage☆
- Non-acute effects of different doses of 3, 4-methylenedioxymethamphetamine on spatial memory in the Morris water maze in Sprague-Dawley male rats**☆●
- Hypothermic intervention for 3 hours inhibits apoptosis in neonatal rats with hypoxic-ischemic brain damage★
- Expression of nerve growth factor precursor, mature nerve growth factor and their receptors during cerebral ischemia-reperfusion injury*☆

