Hypothermic intervention for 3 hours inhibits apoptosis in neonatal rats with hypoxic-ischemic brain damage★
Yale Guo, Zhankui Li, Ruilin Li, Baoshan Su, Shaoping Huang, Jianping Zhou
1Department of Pediatrics, Second Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China
2Department of Neonatology, Maternal and Child Health Hospital of Shaanxi Province, Xi’an 710004, Shaanxi Province, China
3Department of Pathology, Second Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China
INTRODUCTION
Hypoxic-ischemic brain damage (HIBD) is associated with necrosis and apoptosis following ischemia and reperfusion[1-3], and occurs mainly in brain gray and white matter[3-5]. Hypothermia, one of the most promising treatments for protecting the brain from HIBD[6-7], appears to inhibit caspase-3 to reduce apoptosis[1,8-9]. It also appears to involve cell cycle proteins[10]. However, the signaling mechanisms responsible for hypothermia-induced protection are not known and it is unclear if it can simultaneously limit apoptosis in both cortical gray and white matter[11-13].
It is known that the neuroprotective effect of hypothermia is dependent on the choice of time window[14]. However, the optimal duration of hypothermia remains unclear,both in studies of local acute ischemia and in those of hypoxic-ischemic encephalopathy. In research over the last 20 years, various periods of hypothermia have been studied, including 0.5-1 hour, 0.5-5 hours, 1-3 hours, 24 hours, and 12-48 hours[14]. While longer periods of hypothermic intervention were associated with better neural tissue protection, they were also accompanied by severe side effects. Some other protocols would interrupt hypothermic intervention[15].
In this study, we examined damage to gray and white matter in a rat HIBD model using different periods of hypothermia to determine the optimal duration of intervention for premature brains with HIBD.
We also investigated whether neuroprotection was associated with the inhibition of apoptosis and if the cell cycle protein, p16, was involved in this process.
RESULTS
Analysis of experimental animals
A total of 75 Sprague-Dawley rats were randomly divided into five groups: sham surgery group (skin incision and common carotid artery dissociation); model group(HIBD); hypothermia for 3 hours group(HIBD + 31°C for 3 hours); hypothermia for 6 hours group (HIBD + 31°C for 6 hours);and a hypothermia for 15 hours group(HIBD + 31°C for 15 hours)[15]. Each group was subdivided into three subgroups (n = 5)in which hypothermic treatment was commenced either 24, 72 or 168 hours after hypoxia-ischemia. A total of 75 rats were included in the final analysis, without loss.
Scalp temperature of neonatal rats
Meter probes were used every 15 minutes to monitor scalp temperature[16-18]. The temperature of the neonatal rats was stable 15 minutes after the jars had been placed in the water bath. Scalp temperature was 30.8–31.8 °C in the 31 °C water bath,33.8–34.8 °C in the 34 °C water bath and 37.3–38.3 °C in the 37 °C water bath. These met the temperature requirements of the normothermic and hypothermic interventions.
Changes in p16 protein expression and apoptosis
The TdT-mediated dUTP nick end labeling (TUNEL)technique was used to detect apoptosis. The levels of p16, an important cell cycle protein, was examined using immunohistochemistry in the cerebral cortex,hippocampus, and periventricular white matter. The result indicates that the expression of p16 paralleled the extent of apoptosis in the left cortex, hippocampus, and periventricular white matter. Hypothermia at 31 °C for 3 hours, 6 hours and 15 hours decreased p16 expression and apoptosis in brain tissues of neonatal rats with HIBD.
Hypothermia for 3 hours provided the best results(except for p16 in the hippocampus and that of Bcl-2 in the cortex at 24 hours after hypoxia-ischemia; Table 1,Figure 1).
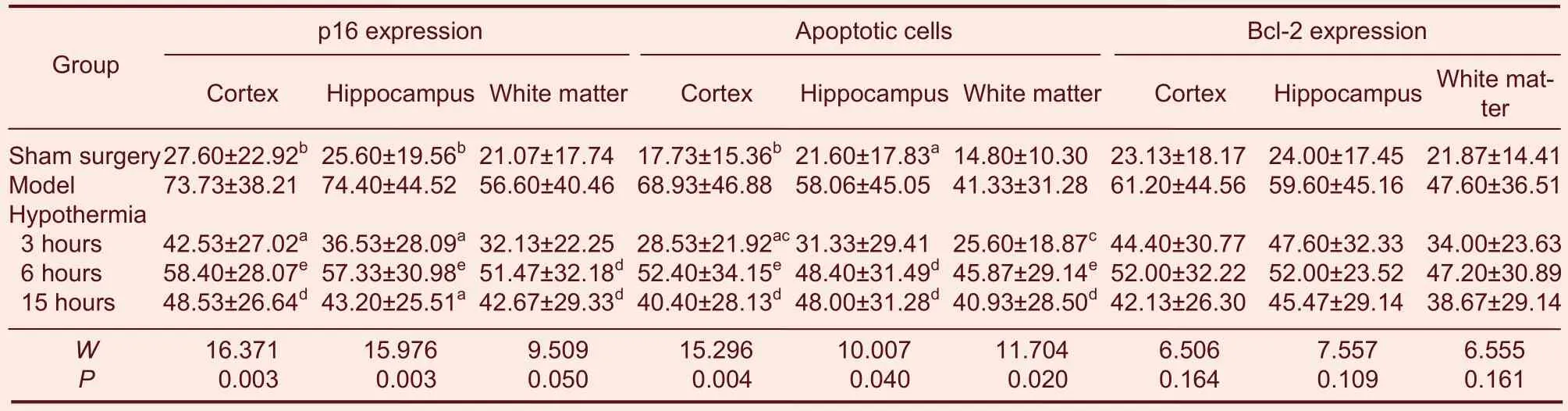
Table 1 Gray values of p16 and Bcl-2 expression, and apoptosis at 24, 72 and 168 hours after hypoxia-ischemia in different brain regions in neonatal rats with hypoxic-ischemic brain damage (HIBD) (gray value)
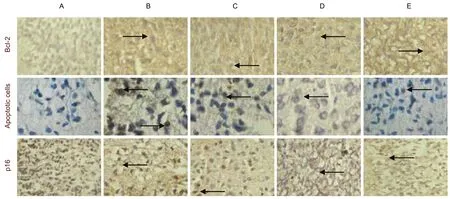
Figure 1 Pathologic changes in p16 (immunohistochemistry staining)expression, apoptosis (TUNEL) and Bcl-2 expression(immunohistochemistry staining) in the cortex of neonatal rats in different brain regions at 24 hours after hypoxia-ischemia(hematoxylin-eosin staining, × 400). (A–E) Sham surgery group, model group, hypothermia for 3 hours group, hypothermia for 6 hours group, hypothermia for 15 hours group, respectively. Brown blue cells (arrows) are p16-positive cells. Brown cells (arrows)are apoptotic cells. Brown yellow cells (arrows) are Bcl-2-positive cells. The cells in sham surgery group were lightly stained; the cells in model group had shrunken and their color was dark; the colors were lighter than that in hypothermia for 3 hours group,hypothermia for 6 hours group or hypothermia for 15 hours group (it was lightest in hypothermia for 3 hours group except in apoptotic cells). The cells in hypothermia for 3 hours group and hypothermia for 6 hours group appeared normal, but the cells in model group and hypothermia for 15 hours group were noticeably damaged, to a similar extent. Measurement data of gray values of Bcl-2 expression are provided in supplementary Table 1 online.
Changes in Bcl-2 protein expression in the brain
Immunohistochemistry was used to examine the expression of Bcl-2, a regulator of apoptosis, in the cerebral cortex, hippocampus and periventricular white matter.
Positive cells were detected in the left hemisphere of each of these regions in every group. There was no significant difference in gray values. Hypothermia at 31 °C for 3, 6 and 15 hours was associated with decreased Bcl-2 expression in the brain of neonatal rats when commenced 24 and 72 hours after hypoxia-ischemia. Hypothermia for 3 hours produced the best results, followed by hypothermia for 15 and 6 hours (Table 1, Figure 1).
Pathology and neural cell counts in the cerebral cortex
Hematoxylin-eosin staining was performed to examine changes in cell morphology and neural cell counts following the various treatments. Necrosis and vacuoles were detected in cells 72 hours after hypoxia-ischemia. There was a noticeable reduction in these indicators of neurodegeneration in the hypothermia for 3 and 6 hours groups, but not in the hypothermia for 15 hours group.
Neural cell counts in the cortex 168 hours after hypoxia-ischemia in the hypothermia for 15 hours group were significantly lower than in the model group (P < 0.01;Table 2), indicating that hypothermia for 15 hours had major side effects.
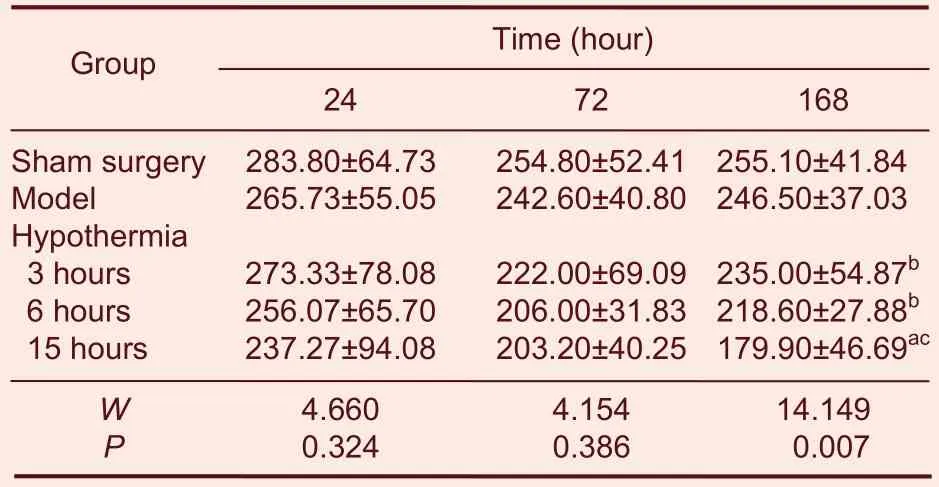
Table 2 Number of neural cells in the cortex at 24, 72 and 168 hours after hypoxia-ischemia (n/mm2)
Correlation of apoptosis and p16 and Bcl-2 expression with cortical neural cell counts
The Spearman correlation test revealed that p16 expression was positively correlated with neural cell numbers in the cortex (r = 0.264, P < 0.01). No significant correlation of Bcl-2 expression or apoptosis to cell number was found (r = 0.188, 0.224, P > 0.05).
DISCUSSION
Simultaneous damage to the cerebral cortex,hippocampus and periventricular white matter of neonatal rats following hypoxia-ischemia
A neonatal rat model of HIBD was designed and implemented in this study. Rats underwent permanent left common carotid artery ligation, and subsequently were given 8% oxygen by inhalation for 3 hours. Rats trembled upon successful completion of the protocol.
Apoptosis increased after hypoxia-ischemia in the cerebral cortex, hippocampus and the periventricular white matter, peaking at 24 hours. Cellular morphology was compromised mainly at 72 hours after hypoxia-ischemia. HIBD was present not only in the gray matter and hippocampus, but also in the white matter,consistent with Gerstner’s observations and his approach, in which the period of hypoxia/ischemia was decreased to 1-3 hours from Rice’s original model. This model has primarily been used in investigations focusing on oligodendrocytes-the white matter was seriously injured if the duration of low oxygen inhalation was 2.5 hours[17-18].
Apoptosis was noticeably increased in the cortex,hippocampus and periventricular white matter in neonatal rats with HIBD in this study, peaking at 24 hours after hypoxia-ischemia. Gray values of p16 and Bcl-2 immunohistochemistry increased concurrently and paralleled the rise in the number of apoptotic cells,indicating that apoptosis contributed to the brain damage in the gray and white matter. Apoptosis was possibly associated with a dysregulated cell cycle, eventually leading to other forms of damage. Thus, a neonatal rat model of HIBD, exhibiting concomitant damage to the gray and white matter could be established by performing permanent left common carotid artery ligation and by providing 8% oxygen inhalation for 3 hours.
Apoptosis was present in both gray and white matter following injury and was associated with increased levels of Bcl-2 and p16.
Protective effects and mechanisms of hypothermic intervention on gray and white matter
Hypothermia inhibits apoptosis to protect the premature brain against HIBD[19-20]. Bcl-2 inhibits apoptosis and p16 regulates apoptosis and the cell cycle. p16 is the main product of the cyclin-dependent kinase 4 inhibitory factor gene and regulates the cell cycle[21]. It remains unclear whether hypothermia protects the gray and white matter simultaneously by inhibiting apoptosis.
7-day-old rats were examined in this study and were treated with hypothermic intervention at 31 °C immediately following hypoxia-ischemia. Hypothermia reduced apoptosis in the cortex, hippocampus and periventricular white matter, and decreased the levels of p16 and Bcl-2. A noticeable decrease in apoptosis occurred if hypothermic intervention at 31 °C was used immediately after surgery, demonstrating protective effects on the brain, which is consistent with a previous study[15]. Hypothermia inhibited apoptosis in the cortex,hippocampus and white matter, indicating that it provides neuroprotection to both gray and white matter. Bcl-2 and p16 staining intensity, as evaluated by gray values, was reduced in the cortex, hippocampus and periventricular white matter in all three groups in this study. Hypothermic intervention at 31°C, either for 3, 6 or 15 hours immediately after hypoxia-ischemia, was neuroprotective by inhibiting apoptosis in both gray and white matter.
Even a short hypothermic intervention of just 3 hours was beneficial.
Optimal duration of hypothermic intervention in neonatal rats with HIBD
A previous study demonstrated that hypothermic interventions of different durations would vary in their neuroprotective effects as well as in their side effects[22],but it was unknown what the optimal duration of intervention was[23-24]. Our results demonstrate that, in neonatal rats with HIBD undergoing 31 °C hypothermia intervention immediately after surgery, hypothermia for 3,6 and 15 hours had brain protective effects, and was associated with reduced p16 expression in the cortex,hippocampus and the periventricular white matter. The decrease in p16 expression was most obvious in the 3-hour hypothermia group, followed by the 15 and 6-hour hypothermia groups. The effects on apoptosis and Bcl-2 expression paralleled those on p16 expression.
Hypothermic intervention at 31°C either for 3, 6 or 15 hours in neonatal rats with HIBD protected the brains by inhibiting the apoptotic process; the effects of 3 and 15-hour interventions were more obvious, while the effect of the 6-hour intervention was the least.
Research into hypothermia intervention for treating hypoxic ischemic encephalopathy has revealed an ever increasing number of side effects[25-26]. Our study revealed that hypothermic intervention at 31°C,immediately after hypoxia-ischemia, reduced apoptosis in the cortex, hippocampus and periventricular white matter in neonatal rats-the effect was most significant in the 15 hours group at 168 hours after hypoxia-ischemia,and the effect was not noticeable either in the 3 or 6 hours group.
How should one choose the optimal hypothermia intervention duration? The 15-hour intervention was not ideal because it reduced neural cell counts in the cortex,and the 3- and 15-hour interventions reduced apoptosis to a greater extent than the 6-hour intervention. Thus, a 3- or 6-hour hypothermic intervention should be chosen.
The reason why hypothermic intervention reduced neural cell counts in the cortex remains poorly understood. We predict that hypothermia intervention for protracted periods leads to more death or poor growth of neural cells.
Of the three different hypothermic intervention durations,the 3-hour treatment appears to be the best choice-it afforded the greatest neuroprotective effect and had the least side effects. The second choice is the 6-hour hypothermic intervention. The 15-hour intervention is not recommended.
In summary, brain damage, including apoptosis in both the gray and white matter, was present after hypoxia-ischemia in neonatal rats, and Bcl-2 and p16 appeared to be involved in this process. The 31°C hypothermic intervention, performed immediately after hypoxia-ischemia, could inhibit apoptosis, demonstrating a protective effect. Hypothermia for 3 hours was optimal,followed by hypothermia for 6 hours. Hypothermia for 15 hours had serious side effects, and thus should not be used.
MATERIALS AND METHODS
Design
A randomized controlled animal study.
Time and setting
This study was performed at the Laboratory of the Department of Pediatrics and the Laboratory of the Department of Pathology, the Second Hospital of the Medical College of Xi’an Jiaotong University, in China,between September 2008 and January 2011.
Materials
A total of 75 healthy Sprague-Dawley rats aged 7 days and weighing 10.2-15.4 g were purchased from the Centre of Animals, Medical Institute, Xi’an Jiaotong University (animal permission No. 08-001). Protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals,formulated by the Ministry of Science and Technology of the People’s Republic of China[27].
Methods
Model establishment
Rat pups underwent permanent left common carotid artery ligation under intraperitoneal injection of 0.25%ketamine (0.1 g/kg), except the sham surgery group[16-17].
The meter probe (M, Y-01 number temperature meter from Medical Meter Factory, Shanghai) was sutured firmly to the scalps of neonatal rats in the sham surgery,model, hypothermia for 3 hours, hypothermia for 6 hours and the hypothermia for 15 hours groups. The rat pups were allowed to recover for 2 hours, and were then provided with 8% oxygen inhalation for 3 hours at 37°C to generate the HIBD model. If the procedure was successful, the rat pup would exhibit seizures[16]. After surgery, the rats in the hypothermia for 3, 6 and 15 hours groups were placed in the 500 mL jars into a water bath at 31°C for 3, 6 and 15 hours, respectively (rats which completed their hypothermic intervention under 15 hours were given a water bath at 37°C to provide a total of 15 hours in the water bath). The rats in the model group were placed in the 500 mL jars into the water bath at 37°C for 15 hours. The meter probes were used every 15 minutes to monitor scalp temperature[18].
Each rat pup was given a lethal injection of 4%paraformaldehyde by heart infusion at 24, 72 or 168 hours after hypoxia-ischemia. The brain was rapidly removed from the skull and embedded in paraffin.
Coronal sections were sliced in 1/3 plane of the thalamus.
Morphology and neural cell counts in the cerebral cortex
Optical microscopy (Leica, Frankfurt, Hesse, Germany)was used to observe cell morphology in the cerebral cortex, hippocampus and periventricular white matter,and neural cells in the cerebral cortex were counted. The number of cells in 1 mm2was counted and expressed as number of cells/mm2.
Immunohistochemical detection of Bcl-2 and p16 expression in the brain
After the antigen was revealed using the microwave, the samples were blocked using normal horse serum(Zhongshan, Beijing, China) for 10 minutes and then incubated with p16 rabbit anti-rat antibody (1: 50) or Bcl-2 rabbit anti-rat antibody (1: 200) in goat anti-rabbit IgG labeled with horseradish peroxidase (1: 1 000) or in goat anti-rabbit IgG and avidin-biotin-peroxidase complex for 1 hour at 37°C. Subsequently, cells were detected using 0.6 mg/mL diaminobenzidine and 0.3%H2O2. The p16-positive cells were stained brown blue,and the Bcl-2-positive cells were stained brown yellow.
The gray values of positive cell expression were analyzed using an image analyzer (Leica)[28-29].
Detection of apoptosis in the rat brain by TUNEL
The TUNEL reagent (Boehringer Mannheim, Mainz,Rheinland-Pfalz, Germany) was used. After paraffin was removed, the samples were incubated in the TUNEL reaction mixture for 1 hour at 37°C, in transforming agent-POD for 15 minutes at room temperature, and then rinsed with PBS three times. Subsequently, cells were detected using 100 μL diaminobenzidine for 5-20 minutes at room temperature. The reaction was ended when a light brown color appeared under the microscope. The samples were counterstained with hematoxylin-eosin, dehydrated, cleared and mounted.
TUNEL staining was then conducted. Positive cells were stained brown. The gray values of apoptotic cells were analyzed using the image analyzer (Leica)[30].
Statistical analysis
Measurement data were expressed as mean ± SD, and enumeration data were expressed as a ratio. SPSS 13.0 software was used for statistical analysis and means were compared using Kruskal-Wallis H detection. The Spearman test was used in correlation analysis between groups. A value of P < 0.05 was considered statistically significant.
Author contributions:Yale Guo participated in study concept and design, performed experiments, analyzed data, and wrote the manuscript. Zhankui Li participated in manuscript writing.Ruilin Li participated in study concept and design. Baoshan Su provided technical support. Shaoping Huang oversaw the experiments. Jianping Zhou participated in statistical analysis.
Conflicts of interest:None declared.
Ethical approval:This study was approved by the Committee of Medical Ethics, the Second Affiliated Hospital of Xi’an Jiaotong University in China.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org and entering Vol. 6, No. 22, 2011 after selecting the “NRR Current Issue” button on the page.
[1]Cotten CM, Shankaran S. Hypothermia for hypoxic–ischemic encephalopathy. Expert Rev Obstet Gynecol. 2010;5(2):227-239.
[2]Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health. 2006;11(5):278-282.
[3]Distefano G, Praticò AD. Actualities on molecular pathogenesis and repairing processes of cerebral damage in perinatal hypoxic-ischemic encephalopathy. Ital J Pediatr. 2010;36(1):63.
[4]Fairchild K, Sokora D, Scott J, et al. Therapeutic hypothermia on neonatal transport: 4-year experience in a single NICU. J Perinatol. 2010;30(5):324-329.
[5]Ryan JC, Morey JS, Bottein MY, et al. Gene expression profiling in brain of mice exposed to the marine neurotoxin ciguatoxin reveals an acute anti-inflammatory, neuroprotective response. BMC Neurosci. 2010;11(8):107.
[6]Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol. 2011;6(9):8-13.
[7]Levine MD, Boyer E. Hyperinsulinemia-euglycemia therapy: a useful tool in treating calcium channel blocker poisoning. Crit Care. 2006;10(4):149.
[8]Okamura T, Ishibashi N, Kumar TS, et al. Hypothermic circulatory arrest increases permeability of the blood brain barrier in watershed Areas. Ann Thorac Surg. 2010;90(6):2001-2008.
[9]Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39-45.
[10]Parikh NA, Lasky RE, Garza CN, et al. Volumetric and anatomic mri for hypoxic-ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments. J Perinatol.2009;29(2):143-149.
[11]Cilio MR, Ferriero DM. Synergistic neuroprotective therapies with hypothermia. Semin Fetal Neonatal Med. 2010;15(5):293-298.
[12]Laing IA. How has research in the last five years changed my clinical practice? Arch Dis Child Fetal Neonatal Ed. 2005;90(5):364-367.
[13]Mullany LC, Katz J, Khatry SK, et al. Neonatal hypothermia and associated risk factors among newborns of southern Nepal. BMC Med. 2010;8(7):43.
[14]Tang XA, Liu LP, Yenari MA. Combination therapy with hypothermia for treatment of cerebral ischemia. J Neurotrauma.2009;26(3):325-331.
[15]Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant.Clin Perinatol. 2009;36(4):859-880.
[16]Peng T, Jia YJ, Wen QQ, et al. Expression of microRNA in neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12(5):373-376.
[17]Gerstner B, Lee J, DeSilva TM, et al. 17beta-estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain. J Neurosci Res. 2009;87(9):2078-2086.
[18]Shintani Y, Terao Y, Ohta H. Molecular mechanisms underlying hypothermia-induced neuroprotection. Stroke Res Treat. 2010;9(8):87-91.
[19]Mietzsch U, Parikh NA, Williams AL, et al. Effects of hypoxic-ischemic encephalopathy and whole-body hypothermia on neonatal auditory function: a pilot study. Am J Perinatol. 2008;25(7):435-441.
[20]Lasky RE, Parikh NA, Williams AL, et al. Changes in the PQRST intervals and heart rate variability associated with rewarming in two newborns undergoing hypothermia therapy. Neonatology.2009;96(2):93-95.
[21]Wong MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol. 2008;164(1-2):28-37.
[22]Liu LP, Yenari MD, Ding YC. Clinical application of therapeutic hypothermia in stroke. Neurol Res. 2009;31(4):331-335.
[23]Kulstad CE, Holt SC, Abrahamsen AA, et al. Therapeutic hypothermia protocol in a community emergency department.West J Emerg Med. 2010;11(4):367-372.
[24]Downs SF, Dyck PC, Rinaldo P, et al. Improving newborn screening laboratory test ordering and result reporting using health information exchange. J Am Med Inform Assoc. 2010;17(1):13-18.
[25]Field DJ, Firmin R, Azzopardi DV, et al. Neonatal ECMO Study of Temperature (NEST)-a randomised controlled trial. BMC Pediatr.2010;10(4):24.
[26]Lobach DF, Kawamoto K, Anstrom KJ, et al. Proactive population health management in the context of a regional health information exchange using standards-based decision support. AMIA Annu Symp Proc. 2007;11(4):473-477.
[27]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[28]Brill J, Huguenard JR. Sequential changes in AMPA receptor targeting in the developing neocortical excitatory circuit. J Neurosci. 2008;28(51):13918-13928.
[29]Kim MS, Seo YK, Park HJ, et al. The neuroprotective effect of recombinant human erythropoietin via an antiapoptotic mechanism on hypoxic-ischemic brain injury in neonatal rats.Korean J Pediatr. 2010;53(10):898-908.
[30]Li Q, Li Z, Xu XY, et al. Neuroprotective properties of picroside II in a rat model of focal cerebral ischemia. Int J Mol Sci. 2010;11(11):4580-4590.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Meta-analysis of transcranial magnetic stimulation to treat post-stroke dysfunction○
- Correlation of E-selectin gene polymorphisms with risk of ischemic stroke A meta-analysis☆
- Penehyclidine hydrochloride attenuates cerebral vasospasm after subarachnoid hemorrhage☆
- Non-acute effects of different doses of 3, 4-methylenedioxymethamphetamine on spatial memory in the Morris water maze in Sprague-Dawley male rats**☆●
- Expression of nerve growth factor precursor, mature nerve growth factor and their receptors during cerebral ischemia-reperfusion injury*☆
- Role of the nerve growth factor precursor-neurotrophin receptor p75 and sortilin pathway on apoptosis in the brain of patients with intracerebral hemorrhage*☆

