Penehyclidine hydrochloride attenuates cerebral vasospasm after subarachnoid hemorrhage☆
Yan Tang, Xiaoyi Chen, Jin Liu
1Laboratory of Anesthesiology and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province,China
2Department of Clinical Medicine, West China Medical School, Sichuan University, Chengdu 610041, Sichuan Province, China
INTRODUCTION
In addition to its cholinoreceptor blocking role, cholinergic antagonists, such as anisodamine, can promote the release of endothelium-derived vascular relaxing factor from the basilar artery in vitro and alleviate cerebral vasospasm after subarachnoid hemorrhage (SAH) in patients[1-3]. However,several side-effects, such as a short half-life and no selectivity to M receptors, limit its potential application. Penehyclidine hydrochloride (PHC), a new cholinergic antagonist developed independently by Chinese scientists, has a long T1/2and is selective for M-receptors. In addition, it is believed that PHC can address the limitations of previous cholinergic antagonists and may play a potential role in the treatment of SAH[4-6]. PHC has been shown to antagonize reactive oxygen species, inhibit lipid peroxidation, modulate the vasomotor function of cerebral vessels,maintain the integrity of endothelial cells and reduce the levels of inflammatory factors[7-10].
Moreover, PHC can prevent cerebral ischemia/reperfusion injury, soman-induced seizures and has been shown to inhibit the pathological increase of aminotransferase in a rat model of cardiopulmonary bypass[11-13].
However, we believe PHC will be more useful in the treatment of SAH. The current study involved monitoring vasospasm histological changes and specific biochemical indicators of brain injury following different routes of PHC administration. In addition, we aimed to determine the efficacy of PHC following administration in a rabbit model of SAH.
RESULTS
Quantitative analysis of experimental animals and grouping
Fifty male New Zealand rabbits were assigned randomly to five groups (n = 10):control (NS group), model (SAH group),intramuscular injection (SAH + IM group),cistern magna injection (SAH + CM group)and saline (SAH + vehicle group). The SAH model was induced by injecting 2 mL of non-heparinized fresh autologous blood from the central artery of the ear into the cisterna magna in the SAH, SAH + IM,SAH + CM and SAH + vehicle groups. The administration protocol involved injecting PHC intramuscularly and 0.3 mL saline into the cisterna magna of rats from the SAH +IM group; 1 mL saline intramuscularly and PHC into the cisterna magna of rats from the SAH + CM group; and 1 mL saline intramuscularly and 0.3 mL saline into the cisterna magna of rats from the SAH +vehicle group at 30 minutes post-SAH. The analepsia time after anesthesia was observed in each animal and the time was transitory. A few rabbits suffered from apnea when liquid was injected into the cistern magna, and approximately 8% of animals had 30 seconds of apnea. Most of these animals had no neurological deficit and only two rabbits from the SAH group had neurological deficit scores of 3 and 4 and one rabbit had a score of 4 in the SAH + CM group(supplementary Table 1 online). One rabbit in the SAM +CM group died at 12 hours post-SAH, but all other animals were used in the final analysis.
General physiological parameters of rabbits before and after SAH
Parameters including heart rate, tympanis temperature,pulse oxygen saturation, and weight pre-SAH and 2 days after injection of PHC in the five groups, were shown to have no significant differences (Table 1). The mean arterial blood pressure (MAP) significantly increased at 0 minute (immediately) and 3 minutes after injection of PHC compared with that of the pre-SAH group (P < 0.01;Figure 1).
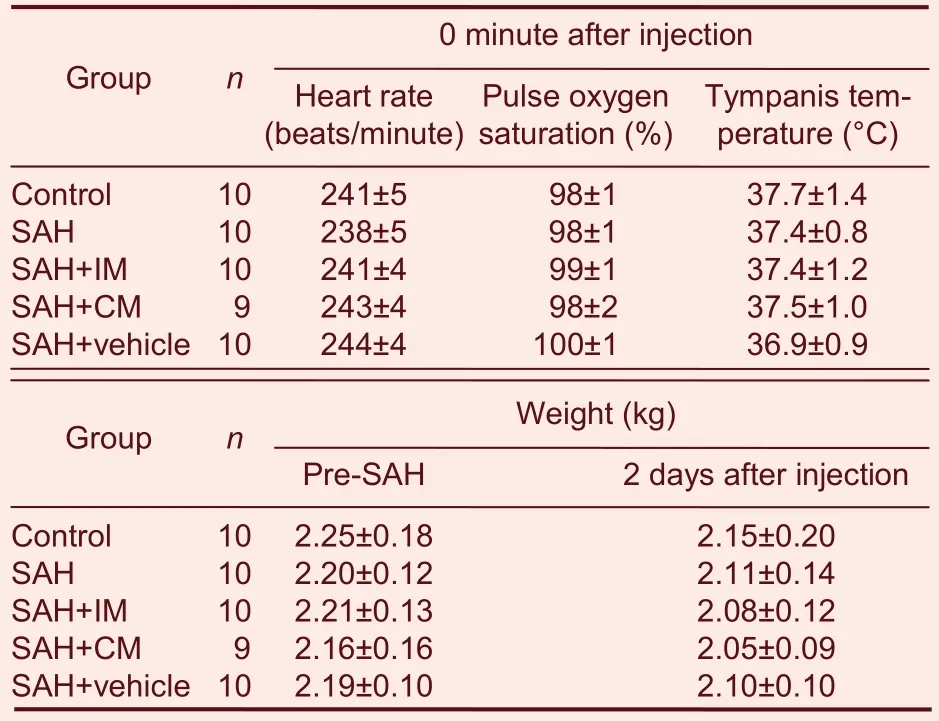
Table 1 Comparison of heart rate, tympanis temperature,pulse oxygen saturation and body weight after injection of penehyclidine hydrochloride
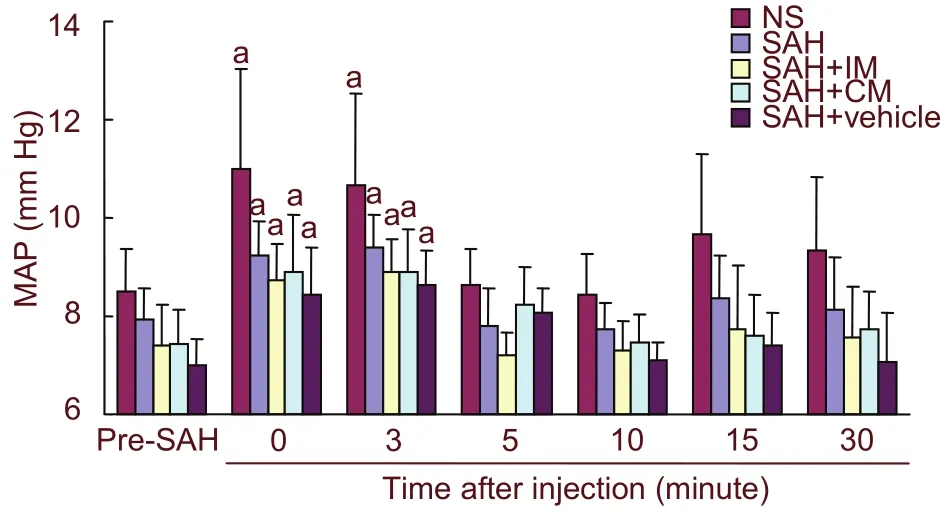
Figure 1 MAP before and after injection of penehyclidine hydrochloride in rabbits. MAP at 0 and 3 minutes after injection was significantly higher than that pre-SAH, while value at 5 minutes after injection was similar to that pre-SAH. aP < 0.05, vs. pre-SAH. Data are expressed as mean ± SD of ten rabbits in each group. Two-way analysis of variance for repeated measurement was used for continuous data for MAP. MAP: Mean arterial blood pressure; SAH: subarachnoid hemorrhage; IM:intramuscular injection; CM: cisterna magna.
Blood gas analysis was measured at 0 minute post-SAH(Table 2). The mean value of arterial blood pH, PO2, PCO2immediately after injection of PHC had no significant difference among the groups. The results reflected stable conditions throughout the injection period after SAH.
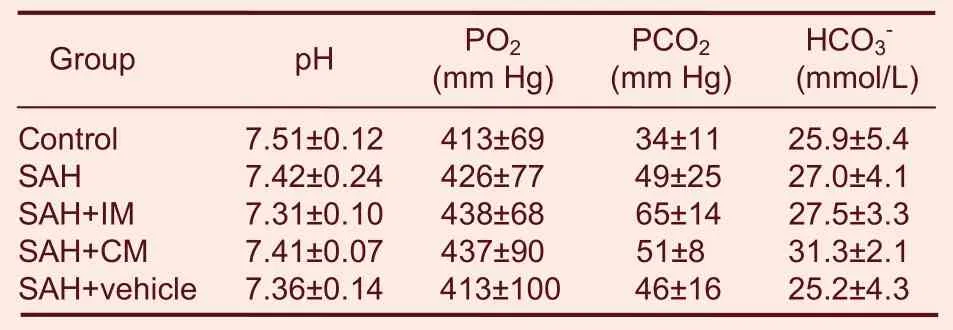
Table 2 Blood gas analysis in every group immediately after SAH
Intramuscular and cisterna magna administration of PHC ameliorated histological and pathological changes in basilar arteries of SAH rabbits
The corrugation coefficient represented the severity of the vasospasm[14]. Light microscopy observations showed that, no shrinkage of the internal elastic lamina was seen in the NS group. Profound vasospasm could be observed in the basilar arteries of rats from the SAH and SAH + vehicle groups at 2 days post-SAH.
Intramuscular and cisterna magna injections of PHC ameliorated the vasospasm in basilar arteries (P < 0.05;Figure 2). The density of surviving neurons in the hippocampus CA1 region was not significantly different among groups (supplementary Table 1 online).
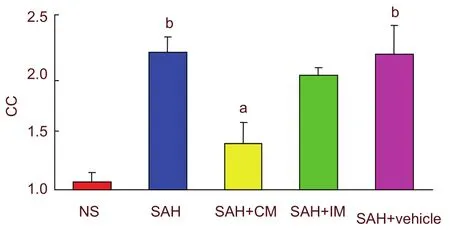
Figure 2 Comparison of corrugation coefficients (CC)before and after injection of penehyclidine hydrochloride.aP < 0.05, vs. SAH group and SAH + vehicle group;bP < 0.05, vs. NS group. One-way analysis of variance(Tukey) and the paired t-test were used to analyze differences among groups. CC = section length/corrugation length (the higher CC value means the more serious vasospasm). SAH: Subarachnoid hemorrhage; IM: intramuscular injection; CM: cisterna magna.
Intramuscular and cisterna magna injections of PHC improved neuron specific enolase (NSE) and S-100β protein expression in cerebrospinal fluid, but did not affect levels of superoxide dismutase (SOD) and malondialdehyde (MDA)
Compared with the NS group, a significant increase in NSE and S-100β protein expression was observed in the SAH and SAH + vehicle groups at 2 days post-SAH(P < 0.05). Intramuscular and cisterna magna injections of PHC significantly decreased NSE and S-100β protein expression (P < 0.05). The effect did not vary according to the injection route (Figure 3).
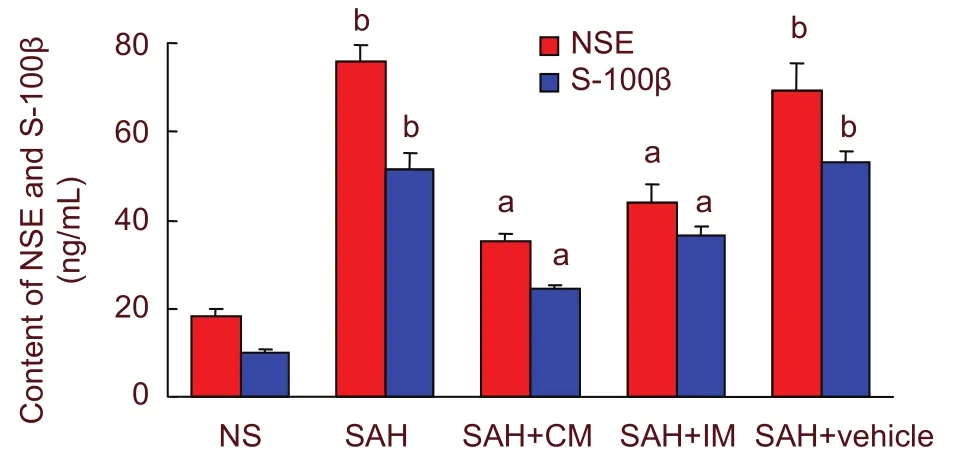
Figure 3 Graphic representation of NSE and S-100β protein expression after intramuscular injection of penehyclidine hydrochloride and injection into the cisterna magna. aP < 0.05, vs. SAH group and SAH + vehicle group; bP < 0.05, vs. NS group. One-way analysis of variance (Tukey) and the paired t-test were used to analyze differences among groups. NSE: Neuron-specific enolase; SAH: subarachnoid hemorrhage; IM:intramuscular injection; CM: cisterna magna.
Compared with the NS group, SOD activity significantly decreased and MDA concentration increased in the cerebrospinal fluid of SAH, SAH + CM, SAH + IM and SAH + vehicle groups at 2 days post-SAH (P < 0.01).However, there was no significant difference among the four groups (P > 0.05; Figure 4).
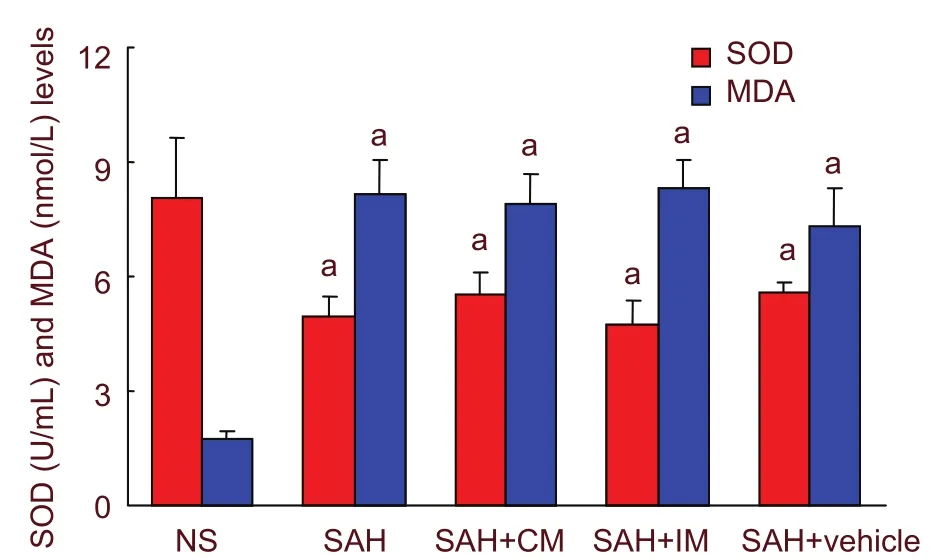
Figure 4 Graphic representation of SOD and MDA levels after intramuscular injection of penehyclidine hydrochloride and injection into the cisterna magna. aP <0.01, vs. NS group. One-way analysis of variance (Tukey)and the paired t-test were used to analyze differences among groups. SOD: Superoxide dismutase; MDA:malondialdehyde; SAH: subarachnoid hemorrhage; IM:intramuscular injection; CM: cisterna magna.
DISCUSSION
In this study, PHC was injected intramuscularly and into the cisterna magna at 30 minutes after model establishment, to avoid unaggregated blood diluting the drug and compromising the drug effect on the target site.
PHC accessed cerebral tissues via the ependyma and prevented vascular vasospasms after SAH. The drug circulated independently of cerebrospinal fluid circulation.These results are in agreement with previous reports[15-16].
The administration of PHC and the interval times were formulated according to drug pharmacokinetic parameters in animal cerebral tissue[17]. In this study, we showed that intramuscular and cisterna magna injections of PHC attenuated basilar artery vasospasm and lowered corrugation coefficient values. We also showed that administration of PHC resulted in a reduction in cerebral vasospasm. In addition, we found that in situ or intravascular administration of PHC had more effective results. Moreover, other experimental studies using PHC have reported its organic protective effects when delivered perivascularly or intraperitoneally[7].
NSE and S-100β are specific biochemical markers that assist in the diagnosis of neuronal injury. A previous study has shown that protein expression of NSE and S-100β increases prior to image examination or pathomorphological measurement. Moreover, higher concentrations are observed at early stages of SAH in cerebrospinal fluid[18]. The experimental results showed that there were no statistical differences in the number of surviving neurons between any two out of the five groups.
Levels of NSE and S-100β protein in the SAH+IM and SAH+CM groups were lower than that of the SAH group(P < 0.01). The reduction in NSE and S-100β protein supports our hypothesis that the new anti-cholinergic drug PHC can prevent neurons from acute injury after SAH. These results could be due to PHC interfering with the self-oxidation reaction of oxyhemoglobin, which is released from blood clots after SAH to induce oxidative stress injury. This stress response results in vasospasm and neurological impairment[19-20]. The experimental results displayed low activity of SOD and high levels of the lipid peroxidation metabolite MDA. The oxygen free radical scavenging properties of PHC, its inhibition of vasospasms, and the neuroprotective effect observed after SAH[21], have been confirmed in clinical and animal studies[22-23]. Although PHC has the capability of scavenging oxygen free radicals, there was no alleviation of oxidative stress after SAH in this study. Further studies are required to verify that a high dose or reduced administration interval of PHC can produce significant neuroprotection.
In conclusion, PHC is a novel self-developed medicine from China that can produce some neuroprotection in the treatment of cerebral vasospasm and cerebral injury in SAH patients. PHC has potential application value in patients with SAH, however its underlying mechanism of action requires further investigation.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
The study was performed at the Laboratory of Anesthesia and Critical Medicine, West China Hospital,Sichuan University, China, from August 2007 to December 2008
Materials
Animals: fifty adult male New Zealand white rabbits of clean grade, weighing 2.0-2.5 kg, were afforded by the Animal Center, College of Basic Medicine, Sichuan University, China, license No. SCXK (Chuan) 2006-0010.
All animals pre-SAH and post-SAH were housed at 23°C and 40-60% humidity. All protocols were approved by the Institutional Animal Care and Use Committee of Sichuan University, China, and conformed to the Guidelines for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH Publication No.85-23, revised 1996).
Drugs: PHC was provided by Chendu Lisite Pharmaceutical Co., Ltd., China. The chemical structural formula of PHC is shown in Figure 5.
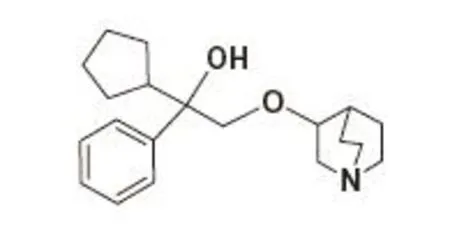
Figure 5 Structure of penehyclidine hydrochloride.
Methods
Establishment of SAH models
The SAH model was established using a modified procedure[24]. After rabbits were weighed, the SAH induction model procedure was performed as follows:Catheters were inserted into veins and artery channels in the rabbits’ ear. After 5-minute oxygen inhalation, rabbits were anesthetized with intravenous administration of ketamine (5 mg/kg), midazolam (1 mg/kg) and pentobarbital sodium (10 mg/kg), sequentially, while in the prone position. The dorsal part of the neck was prepared and sterilized, and cerebrospinal fluid was aspirated under negative pressure when punctured in the atlanto-occipital membrane space with a needle (23G).
Non-heparinized fresh autologous blood from the central artery of the ear (2 mL) was injected rapidly (< 1 minute)into the cisterna magna after the withdrawal of 0.5 mL cerebrospinal fluid. After pressing the local wound for 1 minute, rabbits were kept at a 30° angle with their head positioned downward for 30 minutes. They were then left on their side for 1-2 hours. Each rabbit was returned to its cage and was given food and water, and left at room temperature until righting reflex was regained. In the NS group, 2 mL of saline (37°C) was injected into the cisterna magna.
Intramuscular and cisterna magna injections of PHC
SAH + IM group: PHC was intramuscularly injected at 30 minutes post-SAH into the lateral muscle of the hind leg (0.12 mg/kg, prepared in a 1 mL saline solution; once every 12 hours, for 2 days), and 0.3 mL saline was transfused into the cisterna magna per day for 2 days.
SAH + CM group: PHC was injected into the cistern magna at 30 minutes post-SAH (0.12 mg/kg, prepared in 0.3 mL solution; once per day, for 2 days), 1 mL saline was intramuscularly injected every 12 hours.
SAH + vehicle group: Saline (37°C) was administered at the same intervals via the same route (1 mL saline for intramuscular injection and 0.3 mL saline injected into the cisterna magna).
Detection of physiological parameters in rabbits before and after SAH
Heart rate, oxygen saturation of pulse, and tympanic temperature were determined immediately after SAH.
MAP was also monitored from 0 to 30 minutes post-SAH.Blood gas analysis was measured post-SAH instantly
and 2 days post-SAH.
Neurobehavioral evaluation of SAH rabbits
Neurobehavioral injury score was estimated daily post-SAH using a double blind method[25]. A four point neurological grading scale was used: 1: No neurological deficit (normal); 2: minimum or suspicious neurological deficit; 3: mild neurological deficit without abnormal movement; 4: severe neurological deficit with abnormal movement.
Corrugation coefficient and morphology of neurons in the hippocampal CA1 region
Rabbits were anesthetized with an intravenous injection of pentobarbital sodium (20 mg/kg), and 0.5 mL of cerebrospinal fluid was extracted for detection at 2 days post-SAH. A midsternal thoracotomy was performed rapidly after intravenous anesthesia with pentobarbital(30 mg/kg), and the root of the ascending aorta from the left ventricle apex was cannulated and the right atrium was opened for exsanguinations. Perfusion was performed using saline (500 mL, 37°C), followed by 500 mL of 4% (w/v) paraformaldehyde (10-15°C, 0.2 mol/L in PBS, pH 7.3-7.4) under a pressure of 11.76 kPa. The skull was opened and the blood clot examined. The brainstem, basilar artery and brain tissue were removed and immersed in the fixative liquid for over 12 hours. The fixed brain tissue was cut into 2 to 4 mm thick transverse sections at the chiasm opticum area and fixed.Histological slices (5 μm) were made in the middle basilar artery and then embedded in paraffin. Five sections were sampled randomly, stained with hematoxylin-eosin and the corrugation coefficient was measured using a stereological microscopic analysis system (Olympus, PWS380, Deltapix, Denmark) under a light microscope (400 ×)[26]. The number of surviving neurons with intact cell membranes, full nuclei and clear nucleoli were calculated as the mean number of neurons in the hippocampal CA1 area[27].
Detection of SOD, MDA, NSE and S-100β protein in cerebrospinal fluid of SAH rabbits after PHC injection
The remaining cerebrospinal fluid was centrifuged at 3 000 r/min for 5 minutes at 4°C. The supernatant was aliquoted and stored at -70°C for the measurement of SOD and MDA. SOD activity was detected using the xanthine oxidase method[7]. Briefly, 30 μL of supernatant was added sequentially to reagents according to the manufacturers protocol (Jiancheng Biologic Project Company, Nanjing, China). The mixture was incubated at 37°C for 40 minutes, and a chromogenic agent was added. After 10 minutes, the absorbance was read at 550 nm using an enzyme linked immunosorbent assay(ELISA) reader at room temperature. MDA content was determined using the thiobarbituric acid method. The treated sample mixture (30 μL) was incubated at 95°C for 40 minutes. The cooled mixture was centrifuged at 3 500 r/min for 10 minutes and measured at 532 nm[7].
Levels of S-100β and NSE protein in cerebrospinal fluid were determined using the ELISA method[28]. In brief,samples (80 ng/mL) were diluted in standard diluent substance to a final concentration of 2.5 ng/mL, and blanks (0 ng/mL) and standards (0-80 ng/mL) were prepared for the standard curve. Samples (100 μL) were incubated at 37°C for 1 hour with biotinylated goat anti-rabbit NSE or S-100β protein antibodies (Roche Ltd,USA). Culture plates were rinsed with 0.3 mL buffer solution three times. Residual sample was incubated at 37°C for 30 minutes with streptavidin-horseradish peroxidase solution (Roche). Wells were washed three times with washing buffer and the substrate was added and allowed to incubate at 37°C for 10 minutes (free from illumination). The enzyme-substrate reaction was terminated by pipetting buffer (stopping solution), and the absorbance was immediately read at 450 nm.
Statistical analysis
All data were analyzed using SPSS 15.0 software (SPSS,Chicago, IL, USA) and expressed as mean ± SD.
One-way analysis of variance (Tukey) and the paired t-test were used to analyze differences among groups.Two-way analysis of variance for repeated measurement was used for continuous data for MAP. Statistical significance was set at a P-value < 0.05.
Author contributions:Professor Jin Liu was the mentor for this study. Yan Tang was responsible for funding, conducted the experiments, analyzed the data and wrote the first draft. Xiaoyi Chen contributed to the evaluation and interpretation of this study.
Conflicts of interest:None declared.
Ethical approval:The experimental protocols were approved by Animal Care and Research Committee of Sichuan University in China.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 6, No. 22, 2011 item after selecting the “NRR Current Issue” button on the page.
[1]Poupko JM, Baskin SI, Moore E. The pharmacological properties of anisodamine. J Appl Toxicol. 2007;27(2):116-121.
[2]Song DK, Yang QY, He J. Effect of anisodamine on production of endothelium derived relaxing factor following subarachnoid hemorrhage in rabbit basilar artery and its anti-vasospasm mechanism. Zhongguo Bingli Shengli Zazhi. 1995;11(4):357-361.
[3]Yu H, Yang WL, Ying SB, et al. Intrathecal injection of anisodamine and dexamethasone for cerebral vasospasm after subarachnoid hemorrhage. Nanjing Junyi Xueyuan Xuebao. 2002;24(1):16-17.
[4]Han XY, Liu H, Liu CH, et al. Synthesis of the optical isomers of a new anticholinergic drug, penehyclidine hydrochloride (8018).Bioorg Med Chem Lett. 2005;15(8):1979-1982.
[5]Chen YZ, Zhu HC, Wang XR. The comparison of influences of penehyclidine hydrochloride and atropine on heart rate variability and heart rate in elderly patients. Zhonghua Mazui Xue Zazhi.2005;25(1):59-60.
[6]Yuan SL, Qiao JZ. Pharmacokinetics of penehyclidine hydrochloride in healthy volunteers. Fenxi Ceshi Xuebao. 2001;20(z1):28-29.
[7]Zhan J, Wang Y, Wang C, et al. Protective effects of penehyclidine hydrochloride on septic mice and its mechanism. Shock. 2007;28(6):727-732.
[8]Zhang XF, Pan DB. Effect of penehyclidine hydrochloride on oxidative stress response of myocardium induced by ischemia-reperfusion of both lower limbs of rat. Yixue Linchuang Yanjiu. 2010;27(2):274-278.
[9]Feng SW, Ge TL, Yang JJ, et al. Effect of penehyclidine hydrochloride on lung inflammatory response in septic rats.Zhonghua Mazui Xue Zazhi. 2008;28(1):63-67.
[10]Tu ZZ, Gao J, Chen P. Effects of penehyclidine pretreatment on endotoxin-induced cerebral edema in rats. Chongqing Yike Daxue Xuebao. 2008;33(8):963-966.
[11]Jiang L, Wang JK, Li EY. Effects of penehyclidine hydrochloride pretreatment on transient focal cerebral ischemia/reperfusion injury and expression of caspase-3 in rats. Jilin Daxue Xuebao:Yixue Ban. 2007;33(4):626-630.
[12]Wang YA, Zhou WX, Li JX, et al. Anticonvulsant effects of phencynonate hydrochloride and other anticholinergic drugs in soman poisoning: neurochemical mechanisms. Life Sci. 2005;78(2):210-223.
[13]Cai DS, Jin BB, Pei L, et al. Protective effects of penehyclidine hydrochloride on liver injury in a rat cardiopulmonary bypass model. Eur J Anaesthesiol. 2010;27(9):824-828.
[14]Bunc G, Kovacic S, Strnad S. Evaluation of functional response of cerebral arteries by a new morphometric technique. Auton Neurosci. 2001;93(1-2):41-47.
[15]Marbacher S, Neuschmelting V, Graupner T, et al. Prevention of delayed cerebral vasospasm by continuous intrathecal infusion of glyceroltrinitrate and nimodipine in the rabbit model in vivo.Intensive Care Med. 2008;34(5):932-938.
[16]Tierney TS, Pradilla G, Wang PP, et al. Intracranial delivery of the nitric oxide donor diethylenetriamine/nitric oxide from a controlled-release polymer: toxicity in cynomolgus monkeys.Neurosurgery. 2006;58(5):952-960.
[17]Xue M, Zhang ZQ, Yuan SL, et al. Distribution of penehyclidine hydrochloride raceme and effects of QNB on its four optical isomers in mice brain. Zhongguo Linchung Yaolixue yu Zhiliaoxue.2006;29(9):633-634.
[18]Rasmussen LS, Christiansen M, Eliasen K, et al. Biochemical markers for brain damage after cardiac surgery -- time profile and correlation with cognitive dysfunction. Acta Anaesthesiol Scand.2002;46(5):547-551.
[19]Osuka K, Watanabe Y, Usuda N, et al. Oxidative stress activates STAT1 in basilar arteries after subarachnoid hemorrhage. Brain Res. 2010;1332:12-19.
[20]Lehmann E, Sagher O. Novel treatments for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2008;105:225-228.
[21]Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm.Acta Neurochir Suppl. 2008;104:33-41.
[22]Munakata A, Ohkuma H, Nakano T, et al. Effect of a free radical scavenger, edaravone, in the treatment of patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2009;64(3):423-429.
[23]Güney O, Erdi F, Esen H, et al. N-acetylcysteine prevents vasospasm after subarachnoid hemorrhage. World Neurosurg.2010;73(1):42-49;e3.
[24]Zhou ML, Shi JX, Zhu JQ, et al. Comparison between one- and two-hemorrhage models of cerebral vasospasm in rabbits. J Neurosci Methods. 2007;159(2):318-324.
[25]Jeon H, Ai J, Sabri M, et al. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009;10:103.
[26]Bunc G, Kovacic S, Strnad S. Sympathetic nervous system exclusion following experimental subarachnoid haemorrhage prevents vasospasm in rabbits. Wien Klin Wochenschr. 2000;112(12):533-539.
[27]Kirino T, Tamura A, Sano K. A reversible type of neuronal injury following ischemia in the gerbil hippocampus. Stroke. 1986;17(3):455-459.
[28]H?rdemark HG, Ericsson N, Kotwica Z, et al. S-100 protein and neuron-specific enolase in CSF after experimental traumatic or focal ischemic brain damage. J Neurosurg. 1989;71(5 Pt 1):727-731.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Meta-analysis of transcranial magnetic stimulation to treat post-stroke dysfunction○
- Correlation of E-selectin gene polymorphisms with risk of ischemic stroke A meta-analysis☆
- Non-acute effects of different doses of 3, 4-methylenedioxymethamphetamine on spatial memory in the Morris water maze in Sprague-Dawley male rats**☆●
- Hypothermic intervention for 3 hours inhibits apoptosis in neonatal rats with hypoxic-ischemic brain damage★
- Expression of nerve growth factor precursor, mature nerve growth factor and their receptors during cerebral ischemia-reperfusion injury*☆
- Role of the nerve growth factor precursor-neurotrophin receptor p75 and sortilin pathway on apoptosis in the brain of patients with intracerebral hemorrhage*☆

