Effect of cooling to different sub-zero temperatures on boar sperm cryosurvival
Angelica Garcia-Olivares, Cesar Garzon-Perez, Oscar Gutierrez-Perez, Alfredo Medrano*Facultad de Estudios Superiores Cuautitlán, Unidad de Investigacion Multidisciplinaria. Universidad Nacional Autonoma de Mexico Km .5 Carretera Cuautitlan-Teoloyucan, 5474 Cuautitlan Izcalli, MexicoCentro de Ense?anza, Investigacióny Extensión en Produccion Porcina, Facultad de Medicina Veterinaria y Zootecnia. Universidad Nacional Autonoma de Mexico
ABSTRACT
Objective:To compare different cooling temperatures before ice formation on pig sperm quality, before and after cryopreservation. Methods:Semen diluted in BF5 was cooled from 23 ℃ to 5 ℃ (1% glycerol, 200 ×106cells/mL). Sperm were packaged in plastic straws, and maintained at +5 ℃ per 16 hrs. 1. Freezing point of diluted spermatozoa was determined by exposing straws to nitrogen vapors. 2. Straws (at +5 ℃) were further cooled to -3 ℃, -5 ℃, and -7 ℃, and rewarmed. 3. Straws (at +5 ℃) were further cooled to -3 ℃and -5 ℃, then frozen and stored in liquid nitrogen, and one month later thawed. Progressive motility (PM), viability (Eosin/Nigrosine), plasma membrane functionality (HOST), and acrosome integrity (phase-contrast microscopy) were assessed. Results:1. Freezing point was (-8.2 ± 0.3) (mean ± SEM); one of the ejaculates froze at different temperature from that of the others (P<0.05). 2. PM (%) was 75%, 71%, 63%, and 40% (P<0.05); viability (%) was 90%, 89%, 89%, and 81% (P<0.05); HOST (%) was 49%, 43%, 40%, and 25% (P<0.05); Acrosome integrity (%) was 90%, 89%, 83%, and 81% for +5, -3, -5, and -7 ℃ respectively. 3. PM (%) was 35%, 37%, and 39%; viability (%) was 57%, 60%, and 63%; HOST (%) was 22%, 22%, and 22%; acrosome integrity (%) was 86%, 85%, and 86% for +5, -3, and -5 ℃ respectively. Conclusions:Cooling of pig sperm to -7 ℃ (no freezing) damaged sperm function and structure; in contrast, cooling to either -3 ℃ or -5 ℃ did not change pig sperm survival after freeze-thawing.
ARTICLE INFO
Article history:
Received 10 August 2015
Received in revised form 9 October 2015
Accepted 8 November 2015
Available online 1 January 2016
?
Effect of cooling to different sub-zero temperatures on boar sperm cryosurvival
Angelica Garcia-Olivares1, Cesar Garzon-Perez1, Oscar Gutierrez-Perez2, Alfredo Medrano1*
1Facultad de Estudios Superiores Cuautitlán, Unidad de Investigacion Multidisciplinaria. Universidad Nacional Autonoma de Mexico Km 2.5 Carretera Cuautitlan-Teoloyucan, 54714 Cuautitlan Izcalli, Mexico
2Centro de Ense?anza, Investigacióny Extensión en Produccion Porcina, Facultad de Medicina Veterinaria y Zootecnia. Universidad Nacional Autonoma de Mexico
ABSTRACT
Objective:To compare different cooling temperatures before ice formation on pig sperm quality, before and after cryopreservation. Methods:Semen diluted in BF5 was cooled from 23 ℃ to 5 ℃ (1% glycerol, 200 ×106cells/mL). Sperm were packaged in plastic straws, and maintained at +5 ℃ per 16 hrs. 1. Freezing point of diluted spermatozoa was determined by exposing straws to nitrogen vapors. 2. Straws (at +5 ℃) were further cooled to -3 ℃, -5 ℃, and -7 ℃, and rewarmed. 3. Straws (at +5 ℃) were further cooled to -3 ℃and -5 ℃, then frozen and stored in liquid nitrogen, and one month later thawed. Progressive motility (PM), viability (Eosin/Nigrosine), plasma membrane functionality (HOST), and acrosome integrity (phase-contrast microscopy) were assessed. Results:1. Freezing point was (-8.2 ± 0.3) (mean ± SEM); one of the ejaculates froze at different temperature from that of the others (P<0.05). 2. PM (%) was 75%, 71%, 63%, and 40% (P<0.05); viability (%) was 90%, 89%, 89%, and 81% (P<0.05); HOST (%) was 49%, 43%, 40%, and 25% (P<0.05); Acrosome integrity (%) was 90%, 89%, 83%, and 81% for +5, -3, -5, and -7 ℃ respectively. 3. PM (%) was 35%, 37%, and 39%; viability (%) was 57%, 60%, and 63%; HOST (%) was 22%, 22%, and 22%; acrosome integrity (%) was 86%, 85%, and 86% for +5, -3, and -5 ℃ respectively. Conclusions:Cooling of pig sperm to -7 ℃ (no freezing) damaged sperm function and structure; in contrast, cooling to either -3 ℃ or -5 ℃ did not change pig sperm survival after freeze-thawing.
ARTICLE INFO
Article history:
Received 10 August 2015
Received in revised form 9 October 2015
Accepted 8 November 2015
Available online 1 January 2016
Keywords:
Spermatozoa
Pig
Cooling
Freeze-thawing
Tel:525556231999 ext 39412
E-mail:amedrano@unam.mx
Foundation project:This work was partially supported by Universidad Nacional Autonoma de Mexico:PAPIIT - IT201713 & FES Cuautitlan - PIAPIVC03.
1. Introduction
Boar sperm cryopreservation, producing both good sperm cryosurvival and high on-farm fertility, is still a problem to solve. Two basic protocols for freeze-thawing, with some modifications, are still used[1, 2]. During cryopreservation sperm plasma membrane suffers a series of changes in fluidity due to changes in temperature:when it decreases plasma membrane moves progressively from liquid-crystalline to gel phase, when temperature increases plasma membrane becomes hyper fluid adopting a hexagonal arrangement[3]. Most of the changes associated to cooling occur from 20 ℃ to 0 ℃ but additional phase transitions could take place at sub-zero temperatures[4, 5].
For this, some have proposed to cool down the sperm beyond the traditional cooling temperature (4 to 5 ℃) to allow sperm plasma membrane to accommodate those changes in fluidity without losing selective permeability. Regarding this approach, a number of attempts have been carried out with variable degree of success. Cooling to -2 ℃ or -5 ℃ before freezing has improved cryosurvival of buck, ram and boar spermatozoa[6-8]; in contrast, cooling to -3 ℃ produced no effect on equine sperm cryosurvival[9]. Variation in lipid composition of sperm plasma membrane between animal species[10] could explain those results.
Some protocols for pig sperm cryopreservation, employing freezing machines, have incorporated cooling to -5 or -6 ℃ before ice formation[11, 12]. However, the effect of cooling to other sub-zero temperatures, around the freezing point of common sperm diluents (about -5 ℃), has not been tested.
The objective of this work was to compare the effect of cooling to different sub-zero temperatures, before freezing, on pig sperm cryosurvival.
2. Material and methods
2.1. Semen samples
Semen was collected by the gloved-hand method from 6 boars housed under the same feeding, sanitary and activity conditions; immediately after collection each ejaculate was diluted 1:1 (v/v) with a commercial diluent and transported at about 30 ℃.
2.2. Semen processing
Semen arrived to the laboratory after 90 min approximately, it was left at room temperature to temperate, it was then centrifuged for 10 min at 500×g and supernatant was removed. Pellet was resuspended in BF5 freezing medium[1] without glycerol (400 ×106/mL). One mL of sperm in BF5 was taken and mixed (1:10, v/v) with BTS at 38 ℃, then sperm assessment was carried out.
2.3. Semen assessment
Progressive motility was assessed subjectively under light microscopy; a smear stained by Eosin/Nigrosine (EN) was employed to assess viability and normal/abnormal spermatozoa[13] under light microscopy using the 10× and 20× objectives, 200 cells were counted for each determination.
Sperm plasma membrane functionality was assessed by the hypoosmotic swelling test (HOST) as follows:100 μL of diluted semen were mixed (1:1 v/v) with 100 μL of a hypo-osmotic solution (60 mOsm/kg), this mix was kept in a water bath at 38 ℃ for 30 minutes, and then 30 μL of glutaraldehyde (0.4%) were added to immobilize the spermatozoa; 200 cells were counted under phase-contrast microscopy using the 100× objective.
To assess acrosome integrity, 100 μL of sperm in BTS were taken and mixed (1:1 v/v) with 100 μL of glutaraldehyde (0.4%); percentage of cells showing a smooth and well-defined apical ridge was calculated after counting 200 spermatozoa in phase contrast microscopy using the 100× objective.
Sperm concentration was estimated by counting spermatozoa in the Neubauer chamber employing a dilution 1:200 (sperm:formaldehyde saline solution).
Progressive motility, viability, plasma membrane functionality, and acrosome integrity were assessed before and after (1) coolingrewarming, and (2) freeze-thawing.
2.4. Cooling of spermatozoa
Diluted spermatozoa were slowly cooled from 23 ℃ to 5 ℃ at a rate of 0.04 ℃/min; when diluted sperm reached 7 ℃, BF5 with glycerol was added in three fractions to obtain a final concentration of 200 ×106sperm/mL and 1% glycerol; diluted spermatozoa were packaged in 0.5 mL plastic straws that were sealed with PVA. Straws were put inside glass tubes that were positioned into a special recipient filled with saline water (10% w/v; 500 mL approx.); in this way, straws were kept in vertical position and dry, thus avoiding the stressful step of drying the straws before freezing. That recipient was introduced in a commercial refrigerator. Temperature inside the straws was monitored by means of a digital thermometer (Traceable VWR, Texas USA). Straws were kept at +5 ℃overnight.
2.5. Experimental design
In the first stage, freezing point of diluted spermatozoa was determined by exposing the straws (n=50, 10 per each ejaculate from 5 boars) to nitrogen vapors, 4 cm above the level of liquid nitrogen. Temperature was monitored by means of a thermocouple positioned inside each straw; readouts were saved in a computer. For each frozen straw, the release and the plateau of latent heat of fusion were registered.
In the second stage, straws (at +5 ℃) were further cooled to (1) -3 (0.19 ℃/min), (2) -5 (0.15 ℃/min), and (3) -7 ℃ (0.12 ℃/min), and rewarmed immediately to 38 ℃; straws at +5 ℃ served as control. To cool the straws to sub-zero temperatures, the special recipient employed to carry the straws during cooling from 23 to 5 ℃ into the refrigerator, was introduced into an insulated box filled with crushed saline ice (10% w/v) at -12 ℃; this method has been previously validated in our laboratory. Twelve ejaculates from 6 boars, 3 straws per treatment plus one straw as monitor, were used in this stage.
In the third stage, straws (at +5 ℃) were further cooled to (1) -3 and (2) -5 ℃, frozen in nitrogen vapor 4 cm over liquid nitrogen level for 15 minutes, and stored in liquid nitrogen for at least one month; straws at +5 ℃ served as control. Fourteen ejaculates from 6 boars, 3 straws per treatment plus one straw as monitor, were used in this stage. Straws were thawed by immersion in water at 38 ℃ for 30 seconds; the content of each straw was poured in dry tubes into the water bath.
2.6. Statistical analysis
Data of freezing point was analyzed by the Kruskal-Wallis test to look for possible differences between ejaculates. Data of (1) coolingrewarming and (2) freeze-thawing were analyzed by ANOVA to look for possible differences between cooling treatments. Data expressed as percentages were arcsine transformed to normalize them beforeanalysis. The general linear model procedure from the Statistica for Windows 5.5 software (StatSoft Inc., Tulsa OK, USA, 2000) was used.
3. Results
3.1 First stage - freezing point
Freezing point was (-8.2 ± 0.3) ℃; however, one of the ejaculates froze at different temperature from that of others. The freezing range between straws was -5.1 ℃ to -11.2 ℃ (Table 1).
3.2. Second stage-cooling at different sub-zero temperatures and rewarming
Spermatozoa cooled to +5 ℃, -3 ℃, and -5 ℃ produced similar results for all the assessed variables, but they were different (P<0.05) from those cooled to -7 ℃ (Table 2).
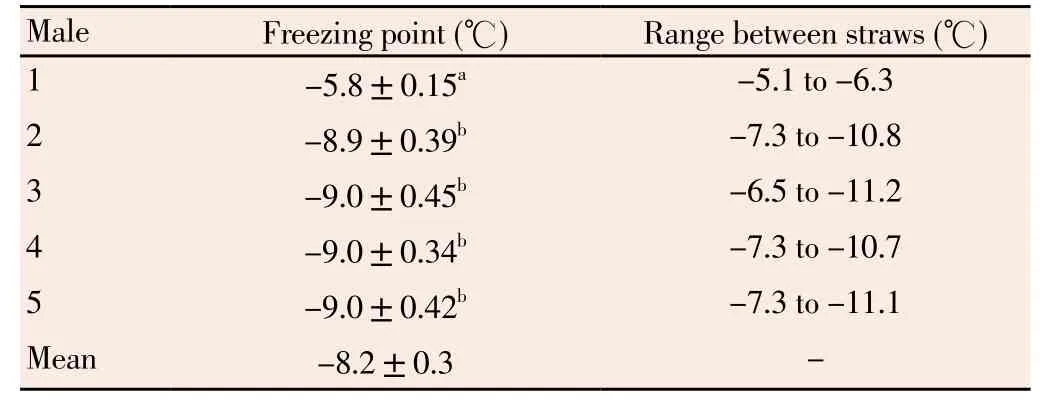
Table 1 Freezing point (℃) of boar spermatozoa diluted in BF5 freezing medium (1% glycerol).
3.3. Third stage-freeze-thawing
Spermatozoa cooled to +5 ℃, -3 ℃, and -5 ℃, before freezing, showed similar values of motility, viability, plasma membrane functionality, and acrosome integrity after thawing (Table 3).
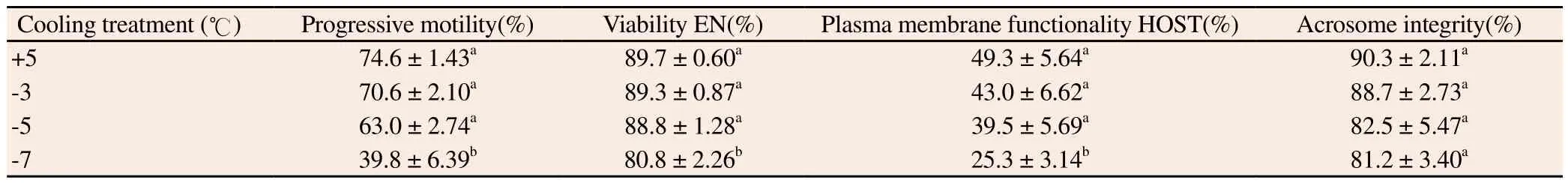
Table 2 Boar sperm quality after cooling to different sub-zero temperatures, and rewarming.

Table 3 Boar sperm quality after cooling to different sub-zero temperatures previous to freeze-thawing.
4. Discussion
The rationale behind cooling of spermatozoa to sub-zero temperatures is to allow plasma membrane to accommodate the changes in its tridimensional structure caused by lipid phase transitions that could take place at those temperatures.
In the first part of this work, freezing point of diluted boar spermatozoa was determined to define a series of target cooling temperatures before ice formation. Thus, in the second part the effect of three sub-zero target temperatures of cooling (-3 ℃, -5 ℃, -7 ℃) on sperm quality at rewarming were assessed. Based on that experiment, two sub-zero target temperatures of cooling (-3 ℃, -5 ℃) were chosen to test their effect on sperm survival after freezethawing.
The difference in the freezing point of one ejaculate with respect to the other four was unexpected since every ejaculate was processed the same way; i. e., ejaculates were diluted in the same commercial medium for transportation, were centrifuged to remove both seminal plasma and extender, and were rediluted in the same batch of the freezing medium keeping the same sperm concentration. It is too early to say there are differences in the freezing point between boars; for this, one would have to freeze many ejaculates from the same male.
The cooling-rewarming experiment showed that cooling to -7 ℃ reduced significantly sperm quality, involving probably the phenomenon of cold shock[5]; also, there was a risk for some straws to freeze before reaching that temperature.
In contrast to previous experiments on cooling of spermatozoa to sub-zero temperatures before freezing (buck, [6]; ram, [7]; boar, [8]), in this work there was no difference between cooling to +5 ℃ and to either -3 ℃ or -5 ℃. It could be that the long period of storage at 5 ℃ before freezing (16 hours) damaged the spermatozoa; thus, the combined effect of that long cold storage and the stress of freeze-thawing could neutralize any positive effect of cooling to sub-zero temperatures on sperm cryosurvival. In support of this point of view, Contreras-Mendez and Medrano[9] reported that cooling of equine spermatozoa to -3 ℃, previously stored at 5 ℃ overnight, produced no effect on sperm cryosurvival. On the other hand, the effect of cooling temperature could depend on plasma membrane fluidity of the spermatozoa from each male[14].
Interestingly, although cooling to different sub-zero temperatures (-3 ℃, -5 ℃) produced no positive effects on sperm quality it did not compromise sperm quality at both rewarming (cooling without freezing) and thawing. In other conditions, this approach could improve sperm quality.
In conclusion, slow cooling to -7 ℃ and rewarming to 38 ℃decreased sperm viability and acrosome integrity; cooling to subzero temperatures, before freezing, did not improve pig sperm cryosurvival.
Acknowledgements
This work was partially supported by Universidad Nacional Autonoma de Mexico:PAPIIT - IT201713 & FES Cuautitlan -PIAPIVC03. We thank Mr. Agustin Gallegos (CEIEPP-FMVZ) for supplying semen samples.
References
[1] Pursel VG, Johnson LA. Freezing of boar spermatozoa:fertilizing capacity with concentrated semen and a new thawing procedure. J Anim Sci 1975; 40:99-102.
[2] Westendorf P, Richter L, Treu H. Deep freezing of boar sperma. Laboratory and insemination results using the Hulsenberger paillete method. Dtsch Tierarztl Wochenschr 1975; 82:261-267.
[3] Hazel JR. Thermal adaptation in biological membranes:Is homeoviscous adaptation the explanation? Annu Rev Physiol 1995; 57:19-42.
[4] Crowe JH, Hoekstra FA, Crowe LM, Anchordoguy TJ, Drobnis E. Lipid phase transitions measured in intact cells with Fourier transform infrared spectroscopy. Cryobiology 1989; 26:76-84.
[5] Watson PF. Recent development and concepts in the cryopreservation of spermatozoa. Reprod Fertil Dev 1995; 7:871-891.
[6] Medrano A, Cabrera F, Gonzalez F, Batista M, Calero P, Quesada E, Gracia A. Slow cooling to -5 C before freezing improves buck sperm cryosurvival. Cryobiology 2001; 43:365-366.
[7] Rios E, Lopez S, Palacios P, Medrano A. Pre-freeze cooling below 0 C improves ram sperm cryosurvival. In:Franca LR, Godinho HP, Henry M, Melo MIV. (eds.) Proceeding of 15th International Congress of Animal Reproduction, Porto Seguro Brazil. 8-12 August 2004, p. 480.
[8] Garzon-Perez C, Flores HF, Medrano A. A simple osmotic stress test to predict boar sperm cryosurvival. CryoLetters 2010; 31:438-444.
[9] Contreras-Mendez A, Medrano A. A comparative study of two cooling protocols on stallion sperm cryosurvival. Andrologia 2015; doi:10.1111/ and.12479. [Epub ahead of print].
[10] Parks JE, Lynch DV. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology 1992; 29:255-266.
[11] Medrano A, Anderson WJ, Millar JD, Holt WV, Watson PF. A custombuilt controlled-rate freezer for small sample cryopreservation studies. CryoLetters 2002; 23:397-404.
[12] Baishya SK, Biswas RK, Kadirvel G, Deka BC, Suresh Kumar, Sinha S, et al. Effect of conventional and controlled freezing method on the post thaw characteristics of boar spermatozoa. Anim Reprod Sci 2014; 149:231-237.
[13] Barth AD, Oko RJ. Abnormal morphology of bovine spermatozoa. Ames IA:Iowa State University Press; 1989, p. 8-18.
[14] Medrano A, Watson PF, Holt WV. Investigation of pig sperm plasma membrane reorganization using progesterone-albumin-fluorescein probes. Asian Pac J Reprod 2012; 1:27-33.
doi:Document heading 10.1016/j.apjr.2015.12.011
*Corresponding author:Alfredo Medrano. Facultad de Estudios Superiores Cuautitlan, Unidad de Investigación Multidisciplinaria. Universidad Nacional Autonoma de Mexico.
 Asian Pacific Journal of Reproduction2016年1期
Asian Pacific Journal of Reproduction2016年1期
- Asian Pacific Journal of Reproduction的其它文章
- Diagnostic and decision-making difficulties:Placenta accreta at nine weeks’gestation
- Male masturbation device for the treatment of premature ejaculation
- Risk factors and adverse perinatal outcomes associated with low birth weight in Northern Tanzania:A registry-based retrospective cohort study
- Analysis of the androgen receptor CAG repeats length in Iranian patients with idiopathic non-obstructive azoospermia
- Returning of cyclicity in infertile Corriedale Sheep with natural progesterone and GnRH based strategies
- Milk supplements in a glycerol free trehalose freezing extender enhanced cryosurvival of boar spermatozoa
