Milk supplements in a glycerol free trehalose freezing extender enhanced cryosurvival of boar spermatozoa
Rukmali Athurupana, Hiroaki Funahashi,*Department of Animal Science, Graduate School of Environmental and Life Science, Okayama University, Okayama, 700-8530, JapanDepartment of Animal Science, Faculty of Agriculture, Okayama University, Okayama, 700-8530, Japan
ABSTRACT
Objective:To evaluate the effect of skim milk and/or coconut milk in a glycerol-free trehalose extender to improve cryosurvival of boar spermatozoa. Methods:Sperm samples were diluted in an egg-yolk-based freezing extender containing 100 mM trehalose and 0.25% Equex STM supplemented with coconut milk or/ and skim milk at 2% or 5% (w/v). Spermatozoa were cryopreserved in 0.5 mL straws and thawed by a rapid transient method (at 70 ℃ for 8 sec) followed with a stabilizing procedure at 39 ℃. Thawed samples were analyzed for motility, viability, high mitochondrial membrane potential (HMMP), and acrosome damage. Results:Even on the presence of egg yolk, motility, HMMP and viability were significantly higher in extender supplemented with 2% skim milk than controls without skim milk (P<0.05). Post-thaw viability significantly improved with the addition of 2% skim milk plus 2% coconut milk as well (P<0.05). Acrosome damage was considerably lower when the extender was supplemented with 2% coconut milk (P<0.05), whereas the benefit was masked in the presence of 2% skim milk. Conclusion:2% skim milk can be used as supplements for a glycerol-free trehalose and egg yolk-based extender to improve post-thaw survival of boar spermatozoa, whereas 2% coconut milk has an effect to protect boar spermatozoa from acrosome damage.
ARTICLE INFO
Article history:
Received 10 October 2015
Received in revised form 20 October 2015
Accepted 22 October 2015
Available online 1 January 2016
?
Milk supplements in a glycerol free trehalose freezing extender enhanced cryosurvival of boar spermatozoa
Rukmali Athurupana1, Hiroaki Funahashi1,2*
1Department of Animal Science, Graduate School of Environmental and Life Science, Okayama University, Okayama, 700-8530, Japan
2Department of Animal Science, Faculty of Agriculture, Okayama University, Okayama, 700-8530, Japan
ABSTRACT
Objective:To evaluate the effect of skim milk and/or coconut milk in a glycerol-free trehalose extender to improve cryosurvival of boar spermatozoa. Methods:Sperm samples were diluted in an egg-yolk-based freezing extender containing 100 mM trehalose and 0.25% Equex STM supplemented with coconut milk or/ and skim milk at 2% or 5% (w/v). Spermatozoa were cryopreserved in 0.5 mL straws and thawed by a rapid transient method (at 70 ℃ for 8 sec) followed with a stabilizing procedure at 39 ℃. Thawed samples were analyzed for motility, viability, high mitochondrial membrane potential (HMMP), and acrosome damage. Results:Even on the presence of egg yolk, motility, HMMP and viability were significantly higher in extender supplemented with 2% skim milk than controls without skim milk (P<0.05). Post-thaw viability significantly improved with the addition of 2% skim milk plus 2% coconut milk as well (P<0.05). Acrosome damage was considerably lower when the extender was supplemented with 2% coconut milk (P<0.05), whereas the benefit was masked in the presence of 2% skim milk. Conclusion:2% skim milk can be used as supplements for a glycerol-free trehalose and egg yolk-based extender to improve post-thaw survival of boar spermatozoa, whereas 2% coconut milk has an effect to protect boar spermatozoa from acrosome damage.
ARTICLE INFO
Article history:
Received 10 October 2015
Received in revised form 20 October 2015
Accepted 22 October 2015
Available online 1 January 2016
Keywords:
Cryopreservation
Sperm
Pigs
Powdered coconut milk
Skim milk
Trehalose
Tel & FAX:+81-86-251-8329
E-mail:hirofun@okayama-u.ac.jp
Foundation project:This research was supported by funds from Okayama University.
1. Introduction
Sperm cryopreservation is the most efficient method for storing boar spermatozoa for a long period, even though their fertilizing ability is still lower than that of fresh or liquid-preserved semen[1]. During the past few years, substantial progress has been made regarding cryopreservation techniques for boar spermatozoa[2]. Adjustment of cooling and re-warming rates to biophysical properties of boar spermatozoa, new sperm package systems and the achievement of accurately consistent freezing of large numbers of samples using programmable freezers have contributed to post-thaw survival rates above 50%, a threshold similar to that used for bull AI-semen[3]. However, these promising results are overshadowed by low conception rates and smaller litters after artificial insemination, limiting the commercial use of cryopreserved boar spermatozoa to a sub-optimal level[4].
Boar spermatozoa are not easy to cryopreserve due to several reasons. Differences in the sperm freezability have also been reported to exist between breeds, within and between boars, between fractions coming from the same ejaculate and even between the seasons[5-8]. Besides, it is well known that sperm from species with low levels of cholesterol in their sperm membranes, such as boar, have decreased tolerances to cold shock, as compared with sperm from species with high levels of cholesterol, like human[9]. Researchers constantly experiment to develop more practical and less-expensive methods to improve cryosurvival of spermatozoa especially from low freezability boars.
In our recent studies, we have demonstrated that non-permeating sugar trehalose can maintain motility, viability, acrosome integrity, HMMP and in vitro penetrability, when boar spermatozoa were frozen in a glycerol-free freezing extender[10] and thawed after rapid transient thawing at 70 ℃ for 8 sec followed by a stabilizingprocedure at 39 ℃ for 52 sec[11].
On the other hand, various milk based extenders have also been proven to be effective for bovine[12,13], dog[14,15], ram[16], buck [17] and equine[18,19] spermatozoa. Most studies have been carried out using whole milk, skim milk or soy milk[12,20] which the western world is mush aware of, but less attention has been paid for coconut milk, which is rich in antioxidants, fat, proteins, minerals and carbohydrates[21,22]. Therefore, the current study was undertaken to examine the effect of skim milk and coconut milk on the cryosurvival of spermatozoa in our glycerol-free trehalose freezing medium using rapid transient thawing method.
2. Materials and methods
2.1. Chemicals and extenders
Unless specified, all the chemicals were purchased from Sigma Aldrich Japan K.K. (Tokyo, Japan). The basic diluents, modified Modena Solution[23], was composed of 152.61 mM glucose, 23.46 mM sodium citrate 2H2O, 11.9 mM NaHCO3, 6.99 mM EDTA-2Na.2H2O, 46.66 mM TRIS, 15.10 mM citric acid and 10 mg/mL gentamicin. Egg yolk based extender (20% hen’s egg yolk in mMS) was used as the cooling extender. Freezing extender consisted of cooling extender supplemented with 0.25% Equex STM? (Nova chemical sales, Inc, Scituate, MA, USA), 100 mM trehalose (Hayashibara Co. Ltd, Okayama, Japan) and skim milk (S) and/or coconut milk (C). Percentages (w/v) of milk supplements and combinations in trehalose extender are as follows:T; freezing extender without milk supplements (Control), TS2; 2% skim milk, TC2; 2% coconut milk, TCS2; 2% coconut and 2% skim milk, TS5; 5% skim milk, TC5; 5% coconut milk, TCS5; 5% coconut and 5% skim milk in trehalose extenders. Stock solution (10%, w/v) of coconut milk was prepared by dissolving 1 g of coconut milk powder (MAGGI coconut milk powder, Nestle, Colombo, Sri Lanka) in 10 mL of prewarmed mMS. It was filtered using a piece of cheesecloth and centrifuged at 700 ×g for 15 min at room temperature. Coconut milk was carefully drawn off below the upper oily layer using a new needle and syringe to a new tube. Stock solution (10%, w/v) of skim milk was prepared by dissolving 1 g of skim milk powder (Wako Pure Chemical Industries, Ltd, Osaka, Japan) in 10 mL of prewarmed mMS. It was centrifuged at 700 ×g for 15 min at room temperature. Supernatant of the skim milk solution was transferred to a new falcon tube. Both solutions were stored at 15 ℃ for further use.
2.2. Semen collection and processing
Semen samples were collected from three individual Berkshire boars (1-3 years old) with excellent fertility scores (supplied by a local AI center). At least three ejaculates were obtained from each boar, and collection was done once a week. The sperm-rich fraction (SRF) from individual ejaculates was collected into a prewarmed tube by gloved-hand technique. Two diluted samples were made by adding SRF to mMS (1:4) before transportation to the laboratory. The semen samples were kept in a Styrofoam box with warm packs (39 ℃) and transported within 1.5 hours. At the laboratory, the samples were assessed for sperm concentration (hemacytometer), viability (SYBR/propidium iodide) and motility (CASA). Sperm samples with >70% of motility and viability were used in the current experiments. SRF was centrifuged at 800 ×g for 5 min at room temperature and 10 mL of the supernatant was diluted in 40 mL of mMS to obtain 20% (v/v) seminal plasma. Previously diluted semen samples were centrifuged at 450 ×g for 5 min at room temperature and the sperm pellet was diluted with 20% seminal plasma to adjust the concentration to 1 × 108cells/mL. Then, it was cooled to 15 ℃in 4 h using a thermo block (ThermoStat plus, Eppendorf, Hamburg, Germany). After incubation at 15 ℃ overnight, sperm sample was washed 3 times with mMS by centrifugation at 620 × g for 5 min at 15 ℃. Then the concentration of the sperm suspension was readjusted to 1 × 109cells/mL with mMS before cryopreservation.
2.3. Cryopreservation of spermatozoa
Sperm samples were suspended in the cooling extender (1:4) at 15 ℃ and cooled down to 5 ℃ over the course of 2 h (ThermoStat plus, Eppendorf, Hamburg, Germany). Then they were resuspended in the freezing extender (1:1) and loaded into precooled 0.5-mL straws (Fujihara Industry Co. Ltd., Tokyo, Japan) while keeping them on ice. Other end of the straw was sealed using a heat-sealer. The straws were frozen by keeping them 4.5 cm above the level of a liquid nitrogen bath (N2 vapor) at approximately -160 ℃ for 15 min. Finally, the straws were plunged into liquid nitrogen (-196 ℃) and stored for 2-3 days until thawing.
2.4. Thawing of frozen spermatozoa
Straws were thawed rapidly and transiently at 70 ℃ for 8 sec and then rapidly maintained at 39 C for 52 sec[11]. Spermatozoa were diluted in prewarmed (39 ℃) mMS (1:2) and washed once by centrifugation (700 × g, 3 min, 39 ℃). Each pellet was resuspended in 1 mL of mMS and post-thaw evaluations were performed after incubating at 39 ℃ for 5 min.
2.5. Evaluation of post-thaw spermatozoa quality
The percentage of total motile spermatozoa was determined using a computer-assisted semen analysis system (CASA, with the Sperm Motility Analysis System software, Digital Image Technology, Tokyo, Japan) with 60 frames per second. For each sample, three subsamples were analyzed, and 2 μL of each subsample was placed on an objective micrometer (Fujihira Industry Co., Ltd., Tokyo, Japan) and a minimum of 300 sperms per subsample were analyzed. Viability, HMMP and acrosome damage was evaluated by fluorescence multiple staining procedure using PI/JC-1/FITC-PNA. Briefly, 3 μL of PI (1 mg/mL), 30 μL of FITC-PNA (200 μg/mL in PBS) and 2 μL of JC-1 (153 μm) were added to 150 μL aliquot of spermatozoa. Next, samples were incubated at 39 ℃ for 8 min inthe dark. Then, 8 μL of the mixture was placed on a glass slide and observed under a fluorescence microscope (× 1 000, Eclipse 80i, Nikon Inc., Tokyo, Japan). A total of 300 sperm cells were counted and the percentage of sperm cells with viability (green head), HMMP (orange-yellow mid piece) and acrosome damage (green acrosome cap) were calculated.
2.6. Statistical analysis
Statistical analyses of results replicated 5 times were used for treatment comparisons and carried out by a one-way ANOVA followed by Tukey’s multiple range test using the GraphPad Prism 6 statistical software (GraphPad Software Inc., La Jolla, CA, USA). All percentage data were subjected to arcsine transformation before statistical analysis if the percentage data contained values less than 10% and/or more than 90%. All data were expressed as the mean ± SEM. Differences were considered significant at P<0.05.
2.7. Experimental design
Sperm samples were cooled in 20% egg yolk extender and frozen with a freezing extender supplemented with 100 mM trehalose, 0.25% (v/v) Equex STM? and milk supplements (skim milk and/ or coconut milk at 2% or 5%). Sperm suspensions were packed into 0.5-ml straws and cryopreserved in liquid nitrogen. Thawed samples were evaluated for motility, viability, HMMP and acrosome damage.
3. Results
The effect of milk supplements added to the glycerol-free trehalose freezing extender on motility, viability, HMMP and acrosome damage of boar spermatozoa after freezing-thawing was evaluated. As shown in Figure 1, when effect of milk supplements in a freezing extender on sperm motility was evaluated by using CASA, addition of 2% skim milk (TS2) in the extender significantly increased the motility [(60.2 ± 3.0) %] as compared with controls [(38.3 ± 4.7)%, P<0.05].
Post-thaw viability of spermatozoa in term of membrane integrity is shown in Figure 2. Percentage of spermatozoa extended in 2% coconut milk plus 2% skim milk [(TCS2, (57.0 ± 2.8) %) and 2% skim milk alone [TS2, (55.6 ± 3.4)%] showed considerably higher viability than those in controls [(40.4 ± 1.6)%, P<0.05].
As shown in Figure. 3, spermatozoa extended in 2 % skim milk (TS2) exhibited a significantly higher percentage of HMMP [(59.0 ± 2.7)%], with orange-yellow multimeric JC-1 aggregates in the mid piece, than those extended in controls [(41.1 ± 0.7)%, P<0.05).
Effect of milk supplements on the acrosome status as evaluated by FITC-PNA, is shown in Figure. 4. Spermatozoa in a trehalose extender containing 2% coconut milk (TC2) exhibited significantly lower acrosomal damage [(9.5 ± 0.7)%] as compared with controls [(17.2 ± 2.0)%, P<0.05].
Higher concentration (5%) of either coconut milk or skim milk was not effective in maintaining any of the post-thaw parameters in boar spermatozoa (P>0.05).
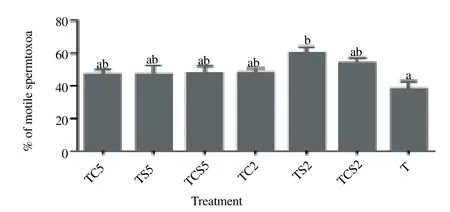
Figure 1. Post-thaw motility of boar spermatozoa frozen in a glycerol-free trehalose-based extender supplemented with skim milk and/or coconut milk. Error bars represent SEM.a,bP<0.05, n=5. T, control without any milk supplements; TS2, 2% (w/v) skim milk; TC2, 2% (w/v) coconut milk; TSC2, 2% skim milk plus 2% coconut milk; TS5, 5% skim milk; TC5, 5% coconut milk; TSC5, 5% skim milk plus 5% coconut milk.
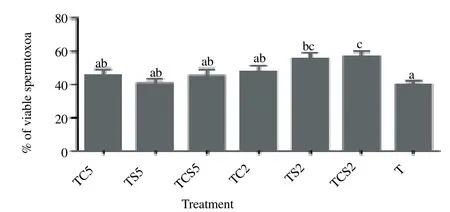
Figure 2. Post-thaw viability of boar spermatozoa frozen in a glycerol-free trehalose-based extender supplemented with skim milk and/or coconut milk. Error bars represent SEM.a,b,cP<0.05, n=5. T, control without any milk supplements; TS2, 2% (w/v) skim milk; TC2, 2% (w/v) coconut milk; TSC2, 2% skim milk plus 2% coconut milk; TS5, 5% skim milk; TC5, 5% coconut milk; TSC5, 5% skim milk plus 5% coconut milk.
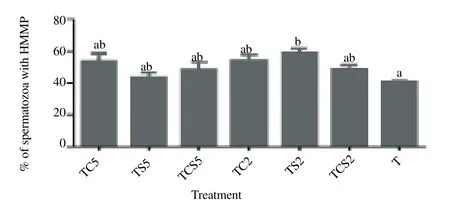
Figure 3. Percentage of spermatozoa with high mitochondria membrane potential (HMMP) after cryopreservation in a glycerol-free trehalose-based extender supplemented with skim milk and/or coconut milk.Error bars represent SEM.a,bP<0.05, n=5. T, control without any milk supplements; TS2, 2% (w/v) skim milk; TC2, 2% (w/v) coconut milk; TSC2, 2% skim milk plus 2% coconut milk; TS5, 5% skim milk; TC5, 5% coconut milk; TSC5, 5% skim milk plus 5% coconut milk.
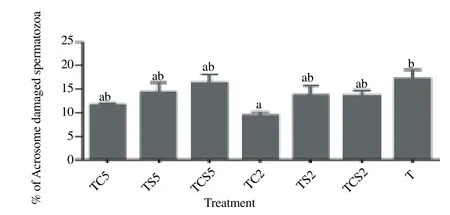
Figure 4. Percentage of spermatozoa with damaged acrosome after cryopreservation in a glycerol-free trehalose-based extender supplemented with skim milk and/or coconut milk.Error bars represent SEM.a,bP<0.05, n=5. T, control without any milk supplements; TS2, 2% (w/v) skim milk; TC2, 2% (w/v) coconut milk; TSC2, 2% skim milk plus 2% coconut milk; TS5, 5% skim milk; TC5, 5% coconut milk; TSC5, 5% skim milk plus 5% coconut milk.
4. Discussion
In the present study, we demonstrated that cryosurvivability of boar spermatozoa was significantly improved by supplementation of a trehalose- egg-yolk-based extender with skim milk and coconut milk. Motility, HMMP and viability were improved with 2% (w/ v) skim milk, regardless of the presence of coconut milk, whereas acrosome damage was reduced with 2% (w/v) coconut milk.
Protective effect of skim milk is provided by the different components in the milk. The foremost protective constituent of milk is most likely to be micelles of caseins, which are the major proteins of milk[24]. It has been reported that skim milk prevent the binding of seminal plasma (SP) proteins to the sperm membrane and reduced sperm lipid loss while maintaining sperm motility and viability [24]. Another study revealed that the LDLs present in an egg-yolkbased extender sequestered the SP proteins avoiding their binding to the sperm membrane, hence preventing the loss of cholesterol and phospholipids in bovine spermatozoa[25]. Since it has been also reported that incorporation of cholesterol into boar, bull or stallion sperm membranes increased sperm tolerance to cryopreservation [26-28], cholesterol in milk may also have an effect to improve the viability after cryopreservation. Skim milk also posses antioxidant properties and[19] Filho et al. reported that dilution of semen with skim milkegg yolk based extender compensates for the loss of protection by the non-enzymatic antioxidant with the removal of seminal plasma. In the present study, in fact, we found that the presence of 2% skim milk in a trehalose-egg-yolk-based extender significantly improved not only viability but also motility and HMMP of boar spermatozoa. Our observation was consistent with previous studies that motility and viability was increased in skim-milk-based extenders in ram and canine spermatozoa, respectively[15,16]. Supplementation of a trehalose- egg-yolk-based extender with 2% skim milk, therefore, seems to be an effective supplement for maintaining the motility and viability of boar spermatozoa after cryopreservation.
In this study, we also found that supplementation of the extender with 2% coconut milk significantly improved the acrosome integrity after cryopreservation. Coconuts are rich in phenols and polyphenols, which are stronger antioxidants than the vitamins[29]. Sperm membranes are rich in polyunsaturated fatty acids and can easily undergo lipid peroxidation in the presence of reactive oxygen species (ROS), leading to changes in membrane fluidity causing oxidative stress[30]. Major protective effect of coconut milk may be due to the antioxidant properties. However, we could not find any significant positive effects of 2% coconut milk on the motility and HMMP of frozen-thawed spermatozoa. In contrast, coconut milk significantly improved sperm motility in buck semen in citrate buffer extender[17] and fertilizing capacity of bovine spermatozoa in an egg yolk based extender[31].
Better motility might be observed if fresh coconut milk or coconut water was used instead of powdered coconut milk. Previous studies reported that deionized coconut water improved the motility, membrane integrity, mitochondria activity and acrosome integrity of boar semen after cryopreservation as compared with filtered coconut water[32]. In a wild animal, collared peccaries (Tayassu tajacu), a coconut water-based extender containing 20% egg yolk and 3% glycerol improved only sperm motility but did not other sperm characteristics[33]. In another wild animal, agouti (Dasiprocta aguti), and dog spermatozoa, a coconut water-based extender has been reported to improve the motility[34]. Together with these evidences, coconut may contain both beneficial and detrimental factors, consequently resulting in different effects dependent on the components of the coconut being used. Although coconut milk has a beneficial effect on sperm characteristics, further research will be required to purify the positive factors in coconut milk. Furthermore, in the present study, the beneficial effects of 2% coconut milk on acrosome integrity and of 2% skim milk on motility and HMMP of spermatozoa were masked in the presence of 2% skim milk or 2% coconut milk, respectively. Therefore, the affinity between skim milk and coconut milk does not appear to be recommended.
In conclusion, skim milk at 2% (w/v) concentration in a glycerolfree trehalose-based extender containing egg yolk improves the motility, viability and HMMP whereas 2% coconut milk has an effect to protect boar spermatozoa from acrosome damage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
Authors would like to thank the Okayama Prefectural Center for Animal Husbandry & Research for providing the semen samples. This research was supported by funds from Okayama University. We also express our gratitude to Prof. Parra, C. M. for her editorial assistance.
References
[1] Rath D, Bathgate R, Rodríguez-Martínez H, Roca J, Strzezek J, Waberski D. Recent advances in boar semen cryopreservation. Soc Reprod Fertil Suppl 2009; 66:51-66.
[2] Didion BA, Braun GD, Duggan MV. Field fertility of frozen boar semen:A retrospective report comprising over 2600 AI services spanning a four year period. Anim Reprod Sci 2013; 137:189-196.
[3] Roca J, Rod iguez-Martínez H, Vázquez JM, Bolarín A, Hernández M, Saravia F, et al. Strategies to improve the fertility of frozen-thawed boar semen for artificial insemination. Soc Reprod Fertil Suppl 2006; 62:261-75.
[4] Rodriguez-Martinez H, Wallgren M. Advances in boar semen cryopreservation. Vet Med Int 2011; 2011:396181.
[5] Holt WV, Medrano A, Thurston LM, Watson PF. The significance of cooling rates and animal variability for boar sperm cryopreservation:insights from the cryomicroscope. Theriogenology 2005; 63:370-382.
[6] Hernández M, Roca J, Ballester J, Vázquez JM, Martínez EA, Johannisson A, et al. Differences in SCSA outcome among boars with different sperm freezability. Int J Androl 2006; 29:583-591.
[7] Pena FJ, Saravia F, Nú?ez-Martínez I, Johannisson A, Wallgren M, Rodríguez Martínez H. Do different portions of the boar ejaculate vary in their ability to sustain cryopreservation? Anim Reprod Sci 2006; 93:101-113.
[8] Barranco I, Ortega MD, Martínez-Alborcia M, Vázquez JM, Martínez E, Roca J. Season of ejaculate collection influences the freezability of boar spermatozoa. Cryobiology 2013; 67:299-304.
[9] Darin-Bennett A, White IG. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology 1977; 14:466-470.
[10] Athurupana R, Takahashi D, Ikoki S, Funahashi H. Trehalose in glycerolfree extender enhances post-thaw survival of boar spermatozoa. J Reprod Dev 2015a; 61:205-210.
[11] Athurupana R, Ioki S, Funahashi H. Rapid thawing and stabilizing procedure improve post-thaw survival and in vitro penetrability of boar spermatozoa cryopreserved with a glycerol-free trehalose-based extender. Theriogenology 2015b; 84(6):940-947.
[12] Singh VK, Singh AK, Kumar R, Atreja SK. Development of soya milk extender for semen cryopreservation of Karan Fries (crossbreed cattle). Cryo Letters 2013; 34:52-61.
[13] Foote RH, Brockett CC, Kaproth MT. Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim Reprod Sci 2002; 71:13-23.
[14] Baran A, Ozdas OB, Sandal AI Ak K. Effects of skim milk and Tris extender on frozen-thawed canine sperm morphology. J Anim Vet Adv 2012; 11:3242-3246.
[15] Rota A, Frishling A, Vannozzi I, Camillo F, Romagnoli S. Effect of the inclusion of skimmed milk in freezing extenders on the viability of canine spermatozoa after thawing. J Reprod Fertil Suppl 2001; 57:377-381.
[16] Kulaksiz R, Cebi C, Akcay E. The effect of different extenders on the motility and morphology of ram sperm frozen or stored at 4 C. Turk J Vet Anim Sci 2012; 36:177-182.
[17] Sule WF, Oyeyemi MO, Akusu MO. Coconut milk - citrate as extender for West African dwarf buck spermatozoa at room temperature. Biokemistri 2007; 19(2):65-73.
[18] Batellier F, Magistrini M, Fauquant J, Palmer E. Effect of milk fractions on survival of equine spermatozoa. Theriogenology 1997; 48:391-417.
[19] Filho ICB, Pederlozzi CD, Sgaravatti AM, Gregory RM, Filho CSD, Jobim MIM, et al. Skim milk-egg based semen extender compensates for non-enzymatic antioxidant activity loss during equine semen cryopreservation Anim Reprod 2009; 6(2):392-399.
[20] Kakar SS, Ganguli NC. Milk as an extender for semen:a review. Indian J Anim Sci 1978; 48:777-790.
[21] Sreeramulu D, Raghunath S. Antioxidant and phenolic content of nuts, oil seeds, milk and milk products commonly consumed in India. Food Nutr Sci 2011; 2:422-427.
[22] Seow CC, Gwee CN. Coconut milk:Chemistry and technology. Int J Food Sci Tech 1997; 32:189-201.
[23] Funahashi H, Sano T. Select antioxidants improve the function of extended boar semen stored at 10 degrees C. Theriogenology 2005; 63:1605-1616.
[24] Bergeron A, Brindle Y, Blondin P, Manjunath P. Milk caseins decreased the binding of major proteins of bovine seminal plasma to sperm and lipid loss from the sperm membrane during sperm storage. Biol Reprod 2007; 77:120-126.
[25] Bergeron A, Crete MH, Brindle Y, Manjunath P. Low-density lipoprotein fraction from hen’s egg yolk decreases the binding of the major proteins of bovine seminal plasma to sperm and prevents lipid efflux from the sperm membrane. Biol Reprod 2004; 70:708-717.
[26] Blanch E, Tomás C, Graham JK, Mocé E. Response of boar sperm to the treatment with cholesterol-loaded cyclodextrins added prior to cryopreservation. Reprod Domest Anim 2012; 47:959-964.
[27] Purdy PH, Graham JK. Effect of cholesterol-loaded cyclodextrin on the cryosurvival of bull sperm. Cryobiology 2004; 48:36-45.
[28] Moore AI, Squires EL, Graham JK. Adding cholesterol to the stallion sperm plasma membrane improves cryosurvival. Cryobiology 2005; 51:241-249.
[29] Vinson AJ, Su XH, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods:Fruits. J Agri Food Chem 2001; 49:5315-5321.
[30] Alvarez JG, Storey BT. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa:its effect on sperm motility. Biol Reprod 1982; 27:1102-1108.
[31] Norman CJ. Survival and fertility of bovine spermatozoa kept at variable temperature in coconut milk extender. J Agric Sc 1962; 59:1803-1807.
[32] Bottini-Luzardo M, Centurion-Castro F, Alfaro-Gamboa M, Ake-Lopez R, Herrera-Camacho J. Effect of addition of coconut water (Cocos nucifera) to the freezing media on post-thaw viability of boar sperm. Trop Anim Health Prod 2012; 45:101-106.
[33] Silva MA, Peixoto GC, Santos EA, Castelo TS, Oliveira MF, Silva AR. Recovery and cryopreservation of epididymal sperm from agouti (Dasiprocta aguti) using powdered coconut water (ACP-109c) and Tris extenders. Theriogenology 2011; 76:1084-1089.
[34] Silva MA, Peixoto GC, Lima GL, Bezerra JA, Campos LB, Paiva AL, et al. Cryopreservation of collared peccaries (Tayassu tajacu) semen using a powdered coconut water (ACP-116c) based extender plus various concentrations of egg yolk and glycerol. Theriogenology 2012; 78:605-11.
doi:Document heading 10.1016/j.apjr.2015.12.010
*Corresponding author:Hiroaki Funahashi, Department of Animal Science, Graduate School of Environmental and Life Science, Okayama University, 1-1-1 Tsushima-Naka, Kita-Ku, Okayama 700-8530 Japan.
 Asian Pacific Journal of Reproduction2016年1期
Asian Pacific Journal of Reproduction2016年1期
- Asian Pacific Journal of Reproduction的其它文章
- Diagnostic and decision-making difficulties:Placenta accreta at nine weeks’gestation
- Male masturbation device for the treatment of premature ejaculation
- Risk factors and adverse perinatal outcomes associated with low birth weight in Northern Tanzania:A registry-based retrospective cohort study
- Analysis of the androgen receptor CAG repeats length in Iranian patients with idiopathic non-obstructive azoospermia
- Returning of cyclicity in infertile Corriedale Sheep with natural progesterone and GnRH based strategies
- Effect of cooling to different sub-zero temperatures on boar sperm cryosurvival
