The neuroimaging of neurodegenerative and vascular disease in the secondary prevention of cognitive decline
Morgan J. Schaeffer, Leona Chan, Philip A. Barber
Abstract Structural brain changes indicative of dementia occur up to 20 years before the onset of clinical symptoms. Efforts to modify the disease process after the onset of cognitive symptoms have been unsuccessful in recent years. Thus, future trials must begin during the preclinical phases of the disease before symptom onset. Age related cognitive decline is often the result of two coexisting brain pathologies: Alzheimer’s disease(amyloid, tau, and neurodegeneration) and vascular disease. This review article highlights some of the common neuroimaging techniques used to visualize the accumulation of neurodegenerative and vascular pathologies during the preclinical stages of dementia such as structural magnetic resonance imaging, positron emission tomography, and white matter hyperintensities. We also describe some emerging neuroimaging techniques such as arterial spin labeling, diffusion tensor imaging, and quantitative susceptibility mapping. Recent literature suggests that structural imaging may be the most sensitive and cost-effective marker to detect cognitive decline, while molecular positron emission tomography is primarily useful for detecting disease specific pathology later in the disease process. Currently, the presence of vascular disease on magnetic resonance imaging provides a potential target for optimizing vascular risk reduction strategies, and the presence of vascular disease may be useful when combined with molecular and metabolic markers of neurodegeneration for identifying the risk of cognitive impairment.
Key Words: amyloid; arterial spin labeling; cognitive decline; dementia; imaging; magnetic resonance imaging; positron emission tomography; quantitative susceptibility mapping;tau; vascular disease; white matter hyperintensities
Introduction
The Alzheimer’s Disease International website describes dementia as a “global epidemic” with greater prevalence rates in lower- and middle-income countries. In wake of the current coronavirus disease 2019 pandemic, it has become clear that individuals with dementia are an especially vulnerable population (Brown et al., 2020). With healthcare and monetary resources spread thin, dementia care may be less of a priority, thus reinforcing the need develop effective and accessible detection and prevention strategies for dementia.Pathological features of dementia, such as changes in brain structure, begin more than 20 years before the onset of clinical symptoms (Bateman et al., 2012). Currently, attempts to treat or modify the disease process at the full-blown disease stage have been largely unsuccessful, including those which were intended to prevent or slow the progression of full-blown dementia from a milder stage (Mehta et al., 2017).One of the reasons these trials fail is because they often begin too late in the disease progression, after the patients have been diagnosed with established dementia (Mehta et al.,2017).
In order to administer disease altering interventions prior to a diagnosis of dementia, clinicians and researchers need to be able to identify those at greatest risk for developing the disease. One such tool for accomplishing this is the use of biomarkers that signal normal biological processes, pathology,or response to intervention. The National Institute of Age-Alzheimer’s Association (NIA-AA) has created a subset of diagnostic criteria specifically for prodromal Alzheimer’s disease (AD) type dementia which incorporates biomarker evidence of AD pathology (Jack et al., 2018). These biomarker criteria are broken down into three groupings: biomarkers of aggregated amyloid-beta (Aβ; A); biomarkers of aggregated tau proteins (T); and neurodegeneration or neuronal injury (N).The AT(N) research criteria proposed by the NIA-AA provides a foundation for identifying individuals who may be at risk for developing dementia, independent of cognitive status.
Mild cognitive impairment (MCI) represents the stage between normal cognition and full-blown dementia where a patient may experience symptoms of cognitive decline but does not meet the criteria for full-blown dementia (Petersen,2004). Currently, all clinical systems of diagnosing MCI share core diagnostic criteria: objective cognitive decline as determined through neuropsychological testing, preserved daily life functioning, and lack of full-blown dementia (Vega and Newhouse, 2014). It has long been proposed that patients with MCI may be a potential target population for secondary dementia clinical trials. While a diagnosis of MCI is a risk factor for developing dementia, there are several limitations to using it as a starting point for preventative interventions.First, the prognosis of MCI patients is heterogenous as the annual conversion rate from MCI to dementia is only 5–10%with the majority of MCI patients not progressing to dementia after 10 years of follow-up according to a meta-analysis of MCI cohorts (Mitchell and Shiri-Feshki, 2009). Additionally,the clinical features of MCI such as declines in memory and attention are also present in normal age-related cognitive decline which may further obscure the predictive value of a diagnosis of MCI. Another explanation for why these trials fail may be because by the time a patient is diagnosed with MCI the neurological and cognitive decline may already be too great to halt or reverse. Thus, preventative interventions for dementia may be most effective at the preclinical stage before symptoms of cognitive decline, including those of MCI, begin to present.
As previously stated, the AT(N) criteria are currently designed to be specific for AD pathology. This is a major limitation because dementia patients often have co-existing pathologies with AD, such as synucleinopathies and vascular pathology,even if they present phenotypically with pure AD (Wang et al., 2012). The pathological heterogeneity of dementia is shown inFigure 1. In the case of vascular pathology, not only do dementia and cerebrovascular disease share co-existing neuropathology, they also share common interactions of modifiable risk factors such as diabetes, hypertension, and obesity (Tariq and Barber, 2018). The existence of cognitive impairment in the presence of vascular disease with or without AD pathology is referred to as vascular cognitive impairment (Graff-Radford, 2019). Several studies have attempted to reduce the risk of developing dementia or cognitive decline by targeting modifiable vascular risk factors such as hypertension. Some examples of antihypertensive medication trials and multi-domain risk reduction trials are summarized inTables 1and2. Some studies such as the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) use cognitive decline as the primary outcome, while others, such as The Systolic Hypertension in Europe (Syst-Eur) use incident dementia as outcome measures. As shown inTables 1and2, there have been mixed findings regarding whether targeting vascular risk factors is successful in reducing risk of cognitive decline and dementia. Biomarkers such as structural and molecular imaging may be incorporated into these types of trials to enrich the sample (i.e., those at greatest risk for developing cognitive decline or dementia) and as potential outcome measures. Thus, it may be worthwhile to target individuals who exhibit modifiable vascular risk factors for early detection and prevention strategies and to also add vascular biomarkers(V) to the AT(N) model for identifying cases of preclinical dementia.
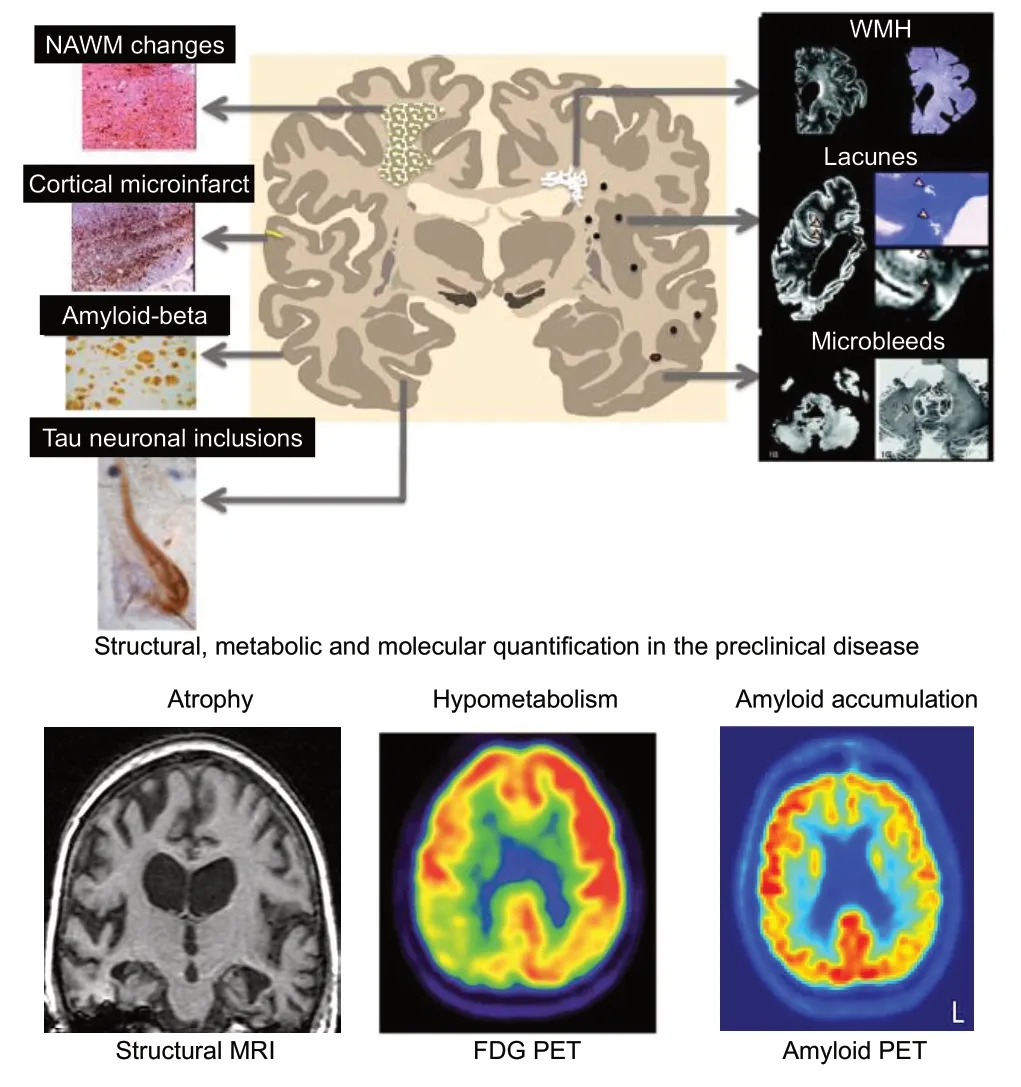
Figure 1|Preclinical detection and quantification of amyloid,neurodegenerative, and vascular diseases.
In this paper, we reviewed the literature regarding the efficacy and practicality of various imaging biomarkers used to identify individuals at risk for dementia. We reviewed not only the established markers for dementia such as those described in the AT(N) but also those vascular biomarkers which are still in the research phase such as white matter hyperintensities and cerebral blood flow. To accomplish this, a review of existing literature of dementia and vascular biomarkers was performed. The references for this biomarker review were found through an electronic search of the PubMed database between April 14, 2020 – May 31, 2020 using a date restriction of 2010–2020. Relevant articles were identified using the following search terms: (“dementia” OR“vascular cognitive impairment” OR “cognitive decline” OR“Alzheimer’s disease” OR “vascular disease”) in combination with (“MRI” OR “structural MRI”) AND (“atrophy” OR “white matter hyperintensities” OR “small vessel disease” OR“arterial spin labeling” OR “diffusion tensor imaging” OR quantitative susceptibility mapping) OR (“PET” OR “amyloid PET” OR “tau PET” OR “FDG-PET”). Titles and abstracts were screened for relevance to the current review. Studies investigating neuroimaging in AD, MCI, and vascular disease were included. Studies investigating biomarkers specific to different subtypes of dementia (e.g., Lewy body dementia)and studies investigating degenerative biomarkers in the context of psychiatric illness (e.g., schizophrenia) were not included. Only articles which were written in English and used human participants were included. Additional articles published before 2010 which were deemed important as well as articles cited only in the context describing techniques and their practical strengths and limitations were selected by the authors.
Magnetic Resonance Imaging
Structural imaging biomarkers
Atrophy of the brain, revealed through structural magnetic resonance imaging (MRI), is a marker of neurodegeneration and neuronal injury according to the AT(N) model of diagnosing AD type dementia (Jack et al., 2018). The NIAAA notes a fundamental difference between markers of neurodegeneration compared to markers of amyloid and tau accumulation: structural MRI markers are not specific to AD pathology. However, Jack and colleagues (2018) do note that structural changes in the brain are perhaps the best indicator of cognitive symptoms in older adults.
Association with cognitive decline and dementia
Recent research investigating the role of cerebral and whole brain atrophy have yielded results that agree with the AT(N)diagnostic framework. Verlinden et al. (2017) found that smaller whole brain volumes were associated with declines in global cognition and activities of daily living in non-demented older adults over a 15-year period. Furthermore, relationships between smaller brain volumes and cognitive decline were exclusively seen in participants who went on to develop dementia, suggesting that a combination of structural and cognitive markers may be a better indicator of disease specific processes rather than structural markers alone. Reduced brain volume in several regions including the medial temporal lobes,cingulate cortex and orbitofrontal cortex occur up to 10 years before cognitive symptoms of MCI or AD manifest (Tondelli et al., 2012).
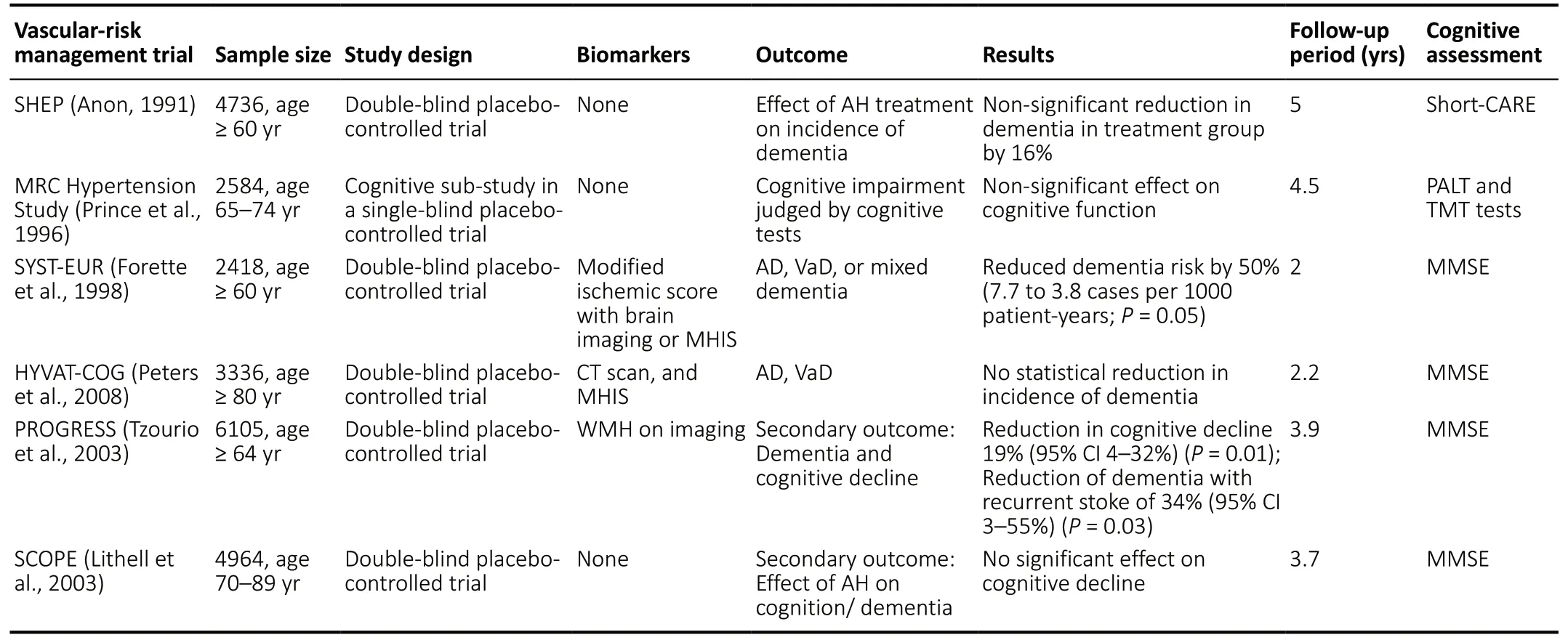
Table 1 |Trials of antihypertensive treatments on dementia and cognitive impairment
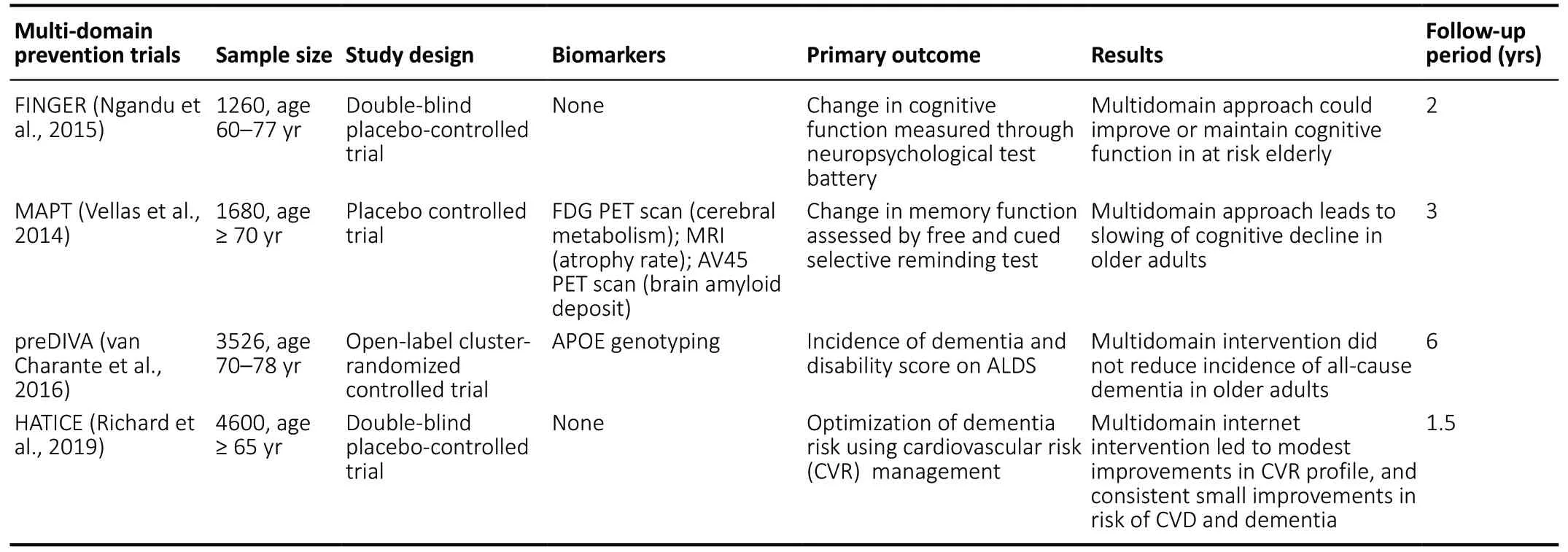
Table 2|Multi-domain preventative trials for dementia and cognitive decline
Atrophy of the hippocampus has been well established as an early biological marker of cognitive decline and dementia due to AD (Ten Kate et al., 2018). Recent studies have continued to find that hippocampal atrophy is a significant predictor for cognitive decline (Haller et al., 2019; Ottoy et al., 2019) and dementia (Van Uden et al., 2015; Korolev et al., 2016; Lambert et al., 2018). Earlier studies found that medial temporal lobe atrophy was a useful biomarker in differentiating dementia due to AD from other dementias as well as predicting amyloid plaques and neurofibrillary tangles, the specific pathological hallmarks of AD (Burton et al., 2009). The findings over the last decades suggest that hippocampal atrophy may be a good biomarker candidate for predicting cognitive decline and dementia in healthy controls as well as MCI patients.
While describing structural atrophy as a marker for cognitive decline and dementia, it is important to distinguish crosssectional volumetric analysis from longitudinal change in cerebral or regional volume (atrophy rates). Cross-sectional brain atrophy measurements have been used to distinguish AD patients from MCI and healthy controls. A recent study investigating the usefulness of hippocampal atrophy in distinguishing controls from AD patients yielded sensitivity and specificity values ranging from 69–100% and 70–87%respectively (Falgàs et al., 2019). Additionally, 28% of clinically diagnosed AD patients were considered to exhibit little to no atrophy of the hippocampus (Persson et al., 2017). Such findings highlight the limited usefulness of cross-sectional atrophy measures as sensitive and specific markers of cognitive decline and dementia.
Longitudinal rates of atrophy measures provide a more powerful predictive tool for cognitive decline. Techniques such as the boundary shift integral (Freeborough and Fox, 1997),which is a measure of absolute change in brain structure by determining the total volume through which a boundary of the cerebrum or regional brain structure (commonly the hippocampus) has moved over time, have been found to be useful in increasing the predictive power of cognitive decline to AD in MCI patients (Leung et al., 2010b). The boundary shift integral is also capable of differentiating between healthy controls and AD patients, with AD patients displaying atrophy rates nearly five times greater than controls (Smith et al., 2007). Additionally, rates of cerebral atrophy correlate strongly with rates of decline in global cognition in patients diagnosed with probable AD (Fox et al., 1999). Rates of medial hippocampal, entorhinal cortex, whole brain, and ventricular atrophy are also capable of differentiating subjects with normal cognition who will decline to MCI or AD or remain stable, with structural changes on MRI being detected more consistently than changes in cognitive assessments (Jack et al., 2004).
In the context of clinical trials which use cerebral atrophy as an outcome, the addition of multiple whole brain measurements over the course of a trial can reduce the required sample size from 32–40% (Schott et al., 2006;Leung et al., 2010a). Similarly, using rates of atrophy, rather than changes in performance on cognitive assessments,substantially reduces the sample size necessary to power an MCI therapeutic trial (Jack et al., 2004). A recent study also noted that in MCI patients, rates of cerebral atrophy, as well as cognitive and functional decline, accelerate well before a patient would test positive for amyloid pathology (Insel et al.,2016). These findings have important implications for future secondary AD prevention trials as many MCI patients may be wrongfully excluded from AD prevention/modification trials if traditional AD amyloid positive thresholds are used as an inclusion criterion. Future clinical trials may wish to use structural neuroimaging in replacement of or in addition to molecular AD biomarkers for determining eligibility.
As previously mentioned, atrophy of the hippocampus is a robust marker of AD dementia as well as cognitive decline.Unfortunately, manual segmentation of the hippocampus is a laborious and time-consuming task, which would limit the utility of such a marker in clinical practice. As such, the use of automated segmentation techniques which can analyze large amounts of data with high accuracy is necessary. Several automated and semi-automated techniques for segmenting the hippocampus currently exist and have been described in detail elsewhere (Dill et al., 2015). These automated techniques typically achieve dice coefficients (accuracy)between 0.70 and 0.90, suggesting that they can segment the hippocampus with reasonable accuracy (Dill et al., 2015)
While MRI acquired structural images are an invaluable tool in dementia biomarker research, sophisticated volumetric analyses often require specialized hardware and software as well as long processing times which may limit its utility as a biomarker in clinical practice. In the case of hippocampal atrophy, a simple, low cost alternative to sophisticated volumetric techniques is the use of visual rating scales such as Scheltons’ medial temporal atrophy (MTA) scores (Scheltens et al., 1992). A recent multi-center study reported similar diagnostic values of MTA scores and hippocampal volumetric analysis, with MTA scores being able to differentiate AD patients from healthy controls with moderate sensitivity (56–72%) and excellent specificity (83–93%) (Harper et al., 2016).Additionally, higher MTA scores (more atrophy) have been associated with faster rates of cognitive decline in a large population-based study (Velickaite et al., 2018).
Associations with vascular disease
Although the atrophy of brain macrostructures is not a direct measure of vascular pathology, recent studies have found relationships between cerebral and whole brain atrophy and vascular disease. For example, O’Brien at al. (2020) found that cardiovascular risk factors of dementia were significant predictors of whole brain atrophy and ventricular expansion over a 2-year period in middle aged adults. However, this change in brain volume may have been mediated through AD pathology, vascular pathology, or a combination of the two.Furthermore, whole brain atrophy rates are accelerated in non-demented patients who have suffered a minor stroke or transient ischemic attack compared with age-matched healthy controls (Munir et al., 2019)
The utility of hippocampal atrophy as a biomarker for cognitive decline in the context of vascular disease and vascular risk factors have yielded mixed results. A recent longitudinal study found that hippocampal atrophy was a significant predictor of progression to dementia in a preclinical cohort for those with less vascular damage and greater memory impairment (Lambert et al., 2018). Participants with vascular dominant pathology who developed dementia had lower rates of hippocampal atrophy, resembling non-converters.Such a finding suggests that sparring of the hippocampus may be useful for ruling out dementia or cognitive impairment predominately caused by vascular disease. However, other studies have found that post-stroke and amyloid positive MCI patients show similar rates of hippocampal atrophy (Selnes et al., 2015).
Summary and limitations
Structural MRI has several advantages over other biomarker tools for cognitive decline and dementia such as being relatively inexpensive and accessible (Ramírez et al., 2019).Additionally, structural neuroimaging is non-invasive making it potentially more appealing to patients and research subjects. While cross-sectional volumetric measurements provide limited value in differentiating AD, MCI, and controls, longitudinal measures of whole brain, cerebral, and hippocampal atrophy have proven to be useful in predicting conversion from MCI or normal cognition to dementia.Furthermore, the utilization of brain atrophy rates in AD clinical trials significantly reduces the sample size necessary to detect small-medium effect sizes.
Although the use of hippocampal segmentation and volumetric techniques may not be widely available in clinical settings, visual rating scores are a simple low-cost alternative which achieve similar levels of sensitivity and specificity for predicting dementia and cognitive decline. However, given the wide variance in the accuracy of hippocampal atrophy for predicting dementia, on their own, hippocampal atrophy may be better utilized as a marker of cognitive impairment(specifically memory decline) rather than a marker of disease specific pathology. The relationship between hippocampal atrophy and cognitive decline is conceptually modeled inFigure 2. Combining structural imaging with the disease specific markers of the AT(N) criteria (amyloid and/or tau)may result in a model with increased sensitivity and specificity for cognitive decline and dementia. This conclusion is in agreement with the NIA-AA AT(N) criteria which note that markers of neurodegeneration and neuronal injury are often not disease specific (Jack et al., 2018).
White matter hyperintensities
White matter hyperintensities (WMH), which are typically detected on fluid-attenuated inversion recovery brain MRI images, are thought to be indicators of small vessel disease(SVD) and are a risk factor for cerebrovascular events such as stroke (Debette and Markus, 2010). WMH are part of the STandards for ReportIng Vascular changes on nEuroimaging(STRIVE) which are a set definitions and standards for image acquisition related to SVD (Wardlaw et al., 2013). Within this framework, WMH of presumed vascular origin are defined as being hyperintense on T2 weighted and fluid-attenuated inversion recovery images, and do not include WMH which are found in other diseases such as multiple sclerosis. Although not currently part of the AT(N) framework for diagnosing AD, WMH may be potential candidate for a vascular (V) type biomarker for dementia to be added in the future.
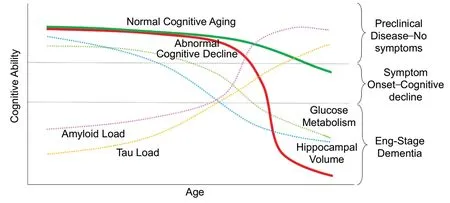
Figure 2|Conceptual diagram showing the relationship between structural, metabolic, and molecular neuroimaging markers and abnormal cognitive decline (red).
Associations with cognitive decline and dementia
Recent studies have found that WMH are associated with cognitive decline and dementia, even independent of established dementia biomarkers such as MR structural imaging and genetic risk factors (Wang et al., 2018; Puzo et al., 2019). A previous systematic review and meta-analysis found excellent sensitivity (95%) though poor specificity (26%)for the diagnosis of vascular or mixed dementia using MRI WMH (Beynon et al., 2012). Despite low disease specificity,WMH are still associated with cognitive decline as a recent population study found that WMH volume at middle age was associated with decline in cognition from childhood to middle adulthood (d’Arbeloff et al., 2019). However, results from the Nun Study found that while cerebrovascular disease increased the risk of developing AD, it did not, on its own impact cognitive status (Mortimer, 2013). Similarly, the Oxford Project to Investigate Memory and Ageing (OPTIMA) found that SVD is only weakly associated with cognitive impairment, and that these associations are overshadowed by the presence of AD pathology (Esiri et al., 2014). Thus, WMH may act to exacerbate cognitive impairment caused by AD pathology rather than directly contributing to cognitive decline.
The relationship between WMH and AD type dementia is less clear. As a marker of SVD, WMH may be indicative of the vascular pathology which often coexists with AD pathology in dementia patients. However, a recent autopsy study found that WMH were associated with AD neuropathology(amyloid plaques and neurofibrillary degeneration) at autopsy,suggesting a potential link between the two (Alosco et al.,2018). Furthermore, WMH are found in familial AD even in the absence of vascular disease (Ryan et al., 2015). Such findings reinforce that WMH are not specific to SVD or AD pathology.Associations with vascular disease
As a marker of small vessel cerebrovascular disease, WMH may be a measure of vascular disease. As previously stated,WMH are found in greater volumes in patients clinically diagnosed with vascular type dementia compared to AD and are a known risk factor for other cerebrovascular diseases such as ischemic stroke. Additionally, WMH are strongly associated with many modifiable vascular risk factors for dementia such as hypertension, fasting glucose blood levels, and visceral fat accumulation (Alber et al., 2019). On the other hand, WMH are increased in adults with familial AD, suggesting that WMH are not specific to vascular disease.
Summary and limitations
As an MRI marker of dementia and cognitive impairment,WMH share many of the same pros as structural neuroimaging(e.g., low cost, non-invasive, etc.). WMH additionally allow for a measure of the vascular contribution to dementia and cognitive decline. However as previously stated, WMH lack disease specificity as they may be markers of pure AD or SVD and are only weakly associated with cognitive decline.As such, WMH provide little clinically useful information on their own. Given this lack of specificity and predictive power,should WMH be added as “V” marker to the AT(N) framework,it would likely be best utilized in combination with other biomarkers in the AT(N) such as structural neuroimaging.Additionally, a recent systematic review found that a wide variety of segmentation techniques exist for determining WMH volume on MRI and only a minority of studies provide a clear definition of WMH (Frey et al., 2019); however this limitation may be overcome in the future if studies adhere to the STRIVE criteria described earlier.
Positron Emission Tomography
Positron emission tomography (PET) can be utilized as a functional or molecular marker for dementia (Ten Kate et al.,2018). Three main PET techniques are utilized in dementia research:18F-fluorodeoxyglucose (FDG) PET, amyloid PET, and more recently, tau PET.
FDG-PET
Associations with cognitive decline and dementia
FDG-PET is a functional marker of dementia which measures cerebral glucose metabolism. Unlike its molecular counterparts, FDG-PET detects glucose metabolism in the brain and therefore is considered a marker of early neurodegeneration within the AT(N) framework (Jack et al., 2018). In amnestic MCI patients, FDG-PET shows a pattern of hypometabolism in the posterior cingulate and parahippocampal regions, and then the temporal, parietal and prefrontal areas as the disease progresses to AD dementia(Shivamurthy et al., 2015). Recent studies have reported excellent sensitivity (86.9–100%) and specificity (82–93.7%) for predicting conversion from MCI to AD, and excellent sensitivity(96%) but suboptimal specificity (54%) for differentiating patients with dementia from controls (F?llmar et al., 2017;Ding et al., 2019; Tripathi et al., 2019). Additionally, FDG-PET has been found to outperform other biomarkers such as MRI volumetry and cerebrospinal fluid (CSF) markers in predicting conversion to AD dementia (Caminiti et al., 2018; Ferrari et al., 2019; Ottoy et al., 2019). Overall, FDG-PET is an excellent marker for predicting conversion from MCI to dementia.
Associations with vascular disease
Despite being an excellent predictor of AD, the association between FDG-PET and vascular disease is less clear. With regards to vascular risk factors for dementia, decreased FDG-PET has been found to be associated with higher blood pressure in middle-aged adults suggesting a link between the two (Langbaum et al., 2012). However, a recent study found that participants who progress from amnestic MCI to vascular dementia show no specific abnormalities in metabolic pattern despite the presence of cortical atrophy on MRI (Tripathi et al., 2019). Furthermore, The European Association of Nuclear Medicine and European Academy of Neurology support the use of FDG-PET in identifying AD patients with vascular pathology only if a pattern of AD hypometabolism is shown and the regions of hypometabolism are not co-localized with cortical infarcts on structural imaging (Nestor et al., 2018).This suggests that FDG-PET may not be useful in detecting vascular pathology in the absence of AD characteristic hypometabolism. Indeed, FDG-PET may be more useful in ruling out AD or detecting comorbid AD pathology in those with vascular disease rather being a direct marker of vascular pathology (Guillén et al., 2020). However, a recent review has suggested that low FDG-PET in AD patients may actually be tracking vascular abnormalities in blood-brain barrier transport (Sweeney et al., 2019), reinforcing that the relationship between vascular pathology and glucose metabolism merits further investigation.
Summary and limitations
FDG-PET has consistently proven to be an excellent predictor of cognitive decline and AD type dementia in those with MCI.Although a pattern of hypometabolism is well established for AD and fronto-temporal dementia, FDG-PET lacks clinical utility for predicting other forms of dementia and discriminating AD from vascular dementia (Nestor et al.,2018; Guillén et al., 2020). Furthermore, FDG-PET involves the injection of a radioactive substance as well as exposure to radiation which may make it a less safe option for some patients compared to other neuroimaging modalities such as MRI. Indeed, emerging functional markers such as arterial spin labeling have recently shown to be comparable to FDGPET in sensitivity and specificity and may provide a safer and more cost-effective alternative (Dolui et al., 2020).
Amyloid PET
Associations with cognitive decline and dementia
As its name suggests, amyloid PET is a technique for visualizing amyloid plaques in the brain. Amyloid PET is a designated as a marker of amyloid pathology within the AT(N) diagnostic framework (Jack et al., 2018). Recent studies have found that amyloid PET is effective at predicting progression to dementia and cognitive decline in adults with normal cognition (Sperling et al., 2019; Timmers et al., 2019) and MCI patients (Ciarmiello et al., 2019). Ciarmiello et al. (2019) report sensitivity and specificity values of 67% and 90% respectively for predicting significant cognitive decline in amnestic MCI patients. For differentiating AD patients from controls, a recent study reported sensitivity and specificity values of 93.7% and 80%respectively (Spallazzi et al., 2019). A recent review notes that quantitative amyloid PET assessment techniques such as standard uptake value ratio achieve higher sensitivity (94.6%vs. 82.3%) and specificity (90.5%vs. 38.1%) for distinguishing demented vs non-demented subjects compared to qualitative assessments (Suppiah et al., 2019). However, such techniques rely on arbitrary thresholds for AD and would likely only be useful as a diagnostic marker rather than a predictive marker in secondary prevention trials.
Associations with vascular disease
Although amyloid PET is not capable of directly assessing vascular pathology, several studies have investigated the relationship between vascular risk factors for dementia and amyloid burden in the brain. For example, the FINGER study (a randomized controlled trial which attempts to prevent cognitive decline in at-risk elderly adults by targeting modifiable vascular and lifestyle risk factors) recently found that participants who were amyloid positive and negative at baseline did not differ with regards to any vascular risk factors such as blood pressure and body mas index(Kemppainen et al., 2018). By contrast, another study found that increased vascular risk factors in midlife, but not late life,were associated with elevated standard uptake value ratio at follow-up approximately 20 years later (Gottesman et al.,2017). Additionally, for participants with vascular risk factors or vascular disease, less cerebral amyloid burden is necessary for cognitive impairment, reinforcing the theory that AD and vascular pathology synergistically contribute to age related cognitive impairment (DeCarli et al., 2019).
Summary and limitations
Amyloid PET imaging is perhaps the best method of visualizing amyloid plaque burden in the brainin vivo. It generally shows good sensitivity and specificity for predicting cognitive decline and dementia in MCI patients and cognitively normal adults,as well as diagnosing AD. Although there is an association with amyloid positivity and cognitive decline, it has been suggested that the rate at which this occurs is dependent on the presence of neurodegeneration (Wirth et al., 2013).Several practical barriers such as the short half-life of some of the isotopes such as the Pittsburgh compound B and the need for a cyclotron which may not be widely available limit the usefulness and practicality of amyloid PET in clinical settings (Shivamurthy et al., 2015). In practice, a number of cut-off scores exist for diagnosing AD, which makes it difficult to standardize just how high the amyloid burden needs to be before an individual is classified as AD or considered at risk for future cognitive decline. Finally, the relationship between amyloid burden on PET and cognitive impairment may be affected by other factors such as cognitive reserve and vascular pathology (Bauckneht et al., 2015; DeCarli et al.,2019), and it is possible for cognitively healthy older adults to be qualitatively scored as “amyloid positive” in clinical settings(Shivamurthy et al., 2015).
Tau PET
Associations with cognitive decline and dementia
Tau PET is a relatively new biomarker for AD. Like amyloid PET, tau PET is a technique for visualizing tau pathology in the brainin vivo, and as such is designated as a marker of aggregated tau within the AT(N) framework (Jack et al.,2018). In MCI patients, tau pathology is generally restricted to the entorhinal cortex region and then expands into cortical regions in AD (Cho et al., 2016). Recent studies have found that increased levels of tau on tau PET are associated with significant cognitive decline in non-demented adults(Aschenbrenner et al., 2018; Sperling et al., 2019), although decline is most apparent in those with both tau pathology and amyloid or neurodegenerative pathology (Jack et al.,2019). In AD patients, Tau PET is strongly correlated with cognitive performance, particularly episodic memory (Teng et al., 2019). Tau PET has also been found to outperform other tau biomarker modalities, namely CSF, in differentiating between the preclinical, prodromal, and full-blown stages of AD dementia (Mattsson et al., 2017). However, Mattsson et al. (2017) reported for each tau stage region, the sensitivity of tau-PET was stronger for full-blown AD (46–100%) than prodromal AD (29–86%) or preclinical AD (0–20%).
Associations with vascular pathology
Like amyloid PET, tau PET is not capable of directly visualizing vascular pathology. However, recent studies investigating the link between tau burden and vascular risk factors of dementia have reported a relationship whereby increased vascular burden is associated with increased tau in non-demented population (Vemuri et al., 2017; Rabin et al., 2019).
Summary and limitations
Despite being a relatively new modality, preliminary evidence suggests that tau PET may be a good candidate biomarker for assessing and predicting cognitive decline in demented and non-demented adults, perhaps even outperforming amyloid PET (Brier et al., 2016; Aschenbrenner et al., 2018).However, as mentioned above, tau PET appears to have better sensitivity for visualizing tau pathology in early stage regions compared to later stage regions and in those farther along in the disease process. Tau PET shares many of its practical limitations with other PET modalities such as the need for a cyclotron which may or may not be clinically available.
Emerging Biomarkers
Arterial spin labeling
Arterial spin labeling (ASL) is a non-invasive MRI technique for visualizing cerebral blood blow (CBF), and is a functional marker of AD (Ten Kate et al., 2018). As a marker of CBF, ASL would be a potential “V” marker to be added to the AT(N)criteria. With regards to vascular pathology, ASL is highly sensitive for small vascular abnormalities that may be missed on structural MRI (Ho, 2018). Recent studies have found that higher CBF is associated with better performance on tasks of memory and executive function, while lower CBF at baseline is associated with subsequent cognitive decline in healthy older adults (Xekardaki et al., 2015; Leeuwis et al., 2018).
Because there is a strong relationship between glucose metabolism and CBF, several studies have directly compared ASL and FDG-PET in dementia prediction studies (Verfaillie et al., 2015). ASL and FDG-PET have similar power in discriminating MCI patients from controls (Dolui et al., 2020).For distinguishing AD patients from controls, ASL has shown superior specificity compared to FDG-PET (84%vs. 54%) but significantly lower sensitivity (53%vs. 96%) (F?llmar et al.,2017). Further research is necessary to standardize ASL in research settings before it can be used in clinical practice.Specifically, there is a lack of longitudinal studies using baseline ASL to predict future dementia or major cognitive decline. However, preliminary evidence suggests that ASL may be a safer, low-cost alternative to FDG-PET.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) detects the diffusion of water molecules in the brain which reflects the integrity of the white matter (Weston et al., 2015). The number of studies utilizing DTI in dementia research is scant, however recent studies have found that DTI is negatively associated with cognitive performance and increased risk of dementia several years before symptoms begin (van Uden et al., 2016; Haller et al.,2019). Despite these associations between DTI and dementia,one study reported that diffusion parameters do not provide additional value for predicting future dementia in a model that already includes WMH and hippocampal volume (Van Uden et al., 2015). Because DTI is sensitive to white matter lesions in patients with SVD (Croall et al., 2017), it is unclear whether DTI would be a marker of neurodegeneration or vascular pathology within the AT(N)V framework.
Quantitative susceptibility mapping
Quantitative Susceptibility Mapping (QSM) is a relatively new technique which enables a quantitative measurement ofin vivodeposition in brain tissue (Haacke et al., 2015).QSM provides an unbiased whole-brain approach to agerelated iron changes. Cerebral iron levels increase with age,cognitive impairment and motor system degeneration (Ward et al., 2014).In vivo, human studies have identified abnormal iron concentrations in neurodegenerative diseases such as AD and Parkinson’s disease, making it a potential marker of neurodegeneration within the AT(N) framework (Ward et al., 2014). There is currently limited data exploring the application of QSM in predicting and monitoring age related cognitive decline, however a recent study found that higher QSM in the hippocampus, temporal, and frontal lobes were associated with cognitive decline in healthy controls and MCI and AD patients with amyloid pathology (Ayton et al., 2017).The authors also noted that QSM levels in the hippocampus may be related to cognitive impairment even in the absence amyloid pathology. Additionally, regions of high iron burden and amyloid burden are colocalized in MCI patients suggesting a direct relationship between these two pathologies (Van Bergen et al., 2016). With regards to vascular disease, iron burden in the hippocampus and putamen are associated with impaired memory and attention respectively in patients diagnosed with subcortical vascular cognitive impairment,which suggests that QSM is sensitive to cognitive decline in multiple pathologies (Sun et al., 2017).
Reflection and Conclusion
The use of biomarkers provides clinicians and researchers a tool for distinguishing individual who may be at risk for major cognitive decline or dementia before symptoms begin.A summary of the biomarkers and their practical uses and limitations are presented inTable 3. A conceptual diagram illustrating the relationship between various neuroimaging markers and abnormal cognitive decline is presented inFigure 2. The results of our review suggest that structural neuroimaging (particularly longitudinal rates of whole brain or hippocampal atrophy) are the most useful tool for predicting cognitive decline and dementia. Despite being less disease specific compared to other A or T markers such as amyloid or Tau PET, structural atrophy may be a useful marker for selecting at risk participants for secondary prevention trials and monitoring outcomes, especially given that amyloid PET may wrongfully exclude potential subjects for these trials due to arbitrary thresholds for amyloid positivity (Insel et al.,2016). Amyloid PET on the other hand may be better utilized as a marker of trial outcome rather than as an inclusion criterion or as a predictor of cognitive decline. A similar model has been proposed elsewhere in which structural neuroimaging is used earlier in the selection process for clinical trials than molecular PET (Ten Kate et al., 2018).
It has been well established that both AD and vascular pathology contribute to the phenotypic profiles of patients diagnosed with dementia, but the value of utilizing vascular biomarkers in secondary prevention is unclear. WMH were the most studied V marker included in this review; however the literature suggests that WMH are only weakly associated with cognition and that they lack disease specificity. Indeed,as shown inTable 2, vascular disease on its own weakly associates with cognitive impairment but may accelerate in the presence of AD pathology. Thus, future studies will need to be conducted to determine the extent to which the addition of WMH improves the prediction of cognitive decline compared to structural markers alone. Preliminary evidence suggests that ASL may be powerful marker for visualizing the vascular contribution to dementia as well as predicting cognitive decline.
An ideal biomarker should be able to predict the symptoms of a disease (e.g., cognitive decline) as well as be specific to the pathology associated with said disease. While no single biomarker is currently capable to fulfilling both criteria mentioned above, a combination of markers from the AT(N)V may succeed in identifying subjects who will decline to dementia with high predictive power and specificity. Future studies will need to be conducted utilizing markers of all four AT(N)V to determine the additional benefit of adding vascular biomarkers to the NIA-AA biological criteria. While a combination of all four biomarker types may be feasible in research settings, future cost-benefit analyses will also need to be conducted to determine whether the added benefit of each biomarker is worth the additional cost.
Acknowledgments:The authors gratefully acknowledge Sandra Black and Christopher Scott at Sunnybrook Health Science Centre for providing the amyloid PET image in Figure 1.
Author contributions:MJS conducted the structured literature review of biomarkers and wrote the manuscript. LC conducted a review of studies investigating the impact of vascular disease on cognitive decline and contributed to the writing of the introduction. PAB conceptualized the topic of the review and provided expert advice on which biomarkers should be included and how the main text should be structured. All authors edited and approved the final manuscript.
Conflicts of interest:None declared.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
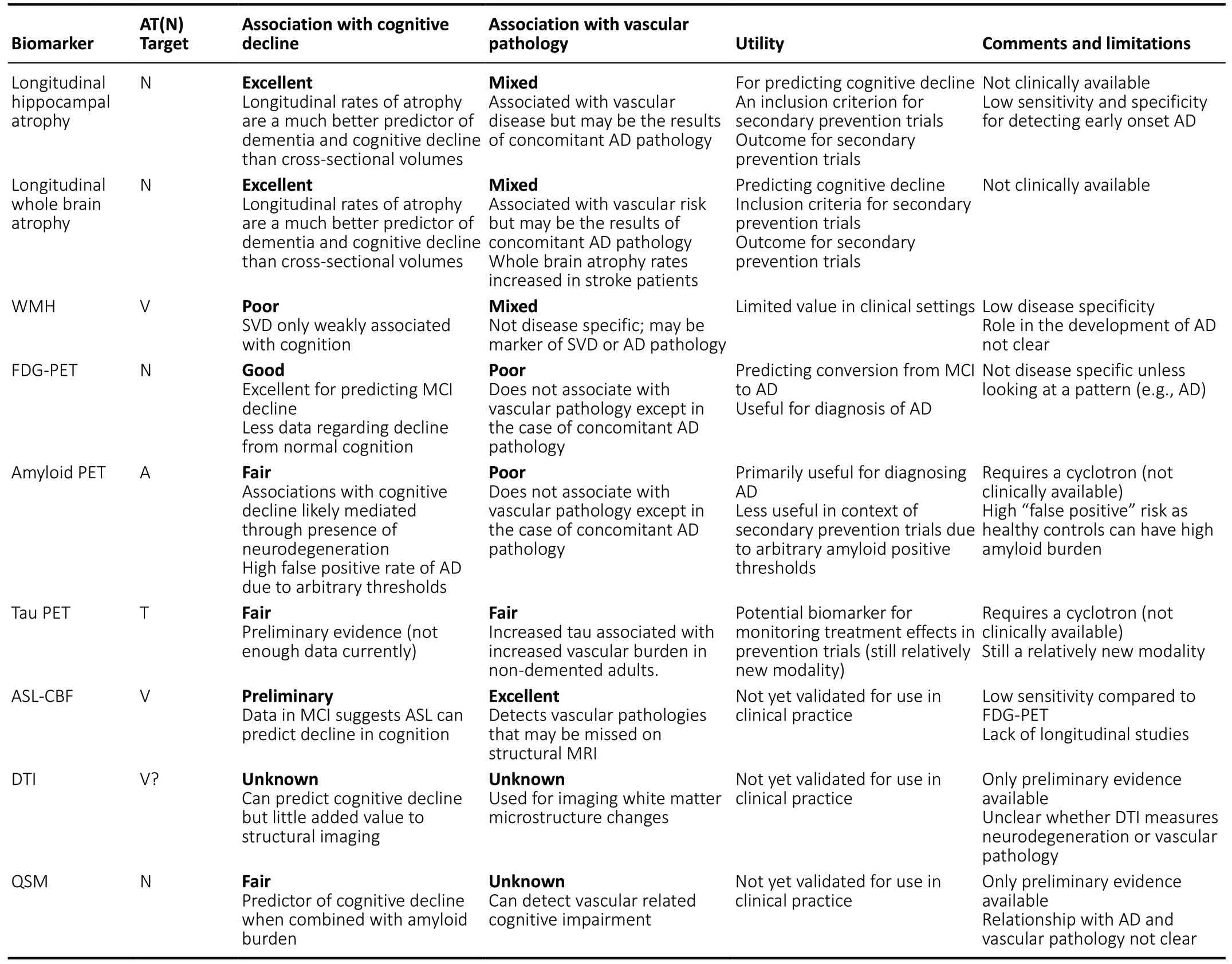
Table 3|Structural, metabolic, and molecular markers of neurodegeneration and vascular disease
- 中國神經(jīng)再生研究(英文版)的其它文章
- Brain-derived neurotrophic factor and its related enzymes and receptors play important roles after hypoxic-ischemic brain damage
- Environmental enrichment combined with fasudiltreatment inhibits neuronal death in the hippocampal CA1 region and ameliorates memory deficits
- Regulation of neuronal bioenergetics as a therapeutic strategy in neurodegenerative diseases
- The secretome of endothelial progenitor cells:a potential therapeutic strategy for ischemic stroke
- Induced pluripotent stem cell technology for spinal cord injury: a promising alternative therapy
- The interaction of stem cells and vascularity in peripheral nerve regeneration