Tongmai Yangxin Pill combined with metoprolol or metoprolol alone for the treatment of symptomatic premature ventricular complex: a multicenter, randomized, parallel-controlled clinical study
Li-Jun LIU, Guo-Hua ZHU, Hong-Yu LUO, Xi-Peng SUN, Jing LI, Qi HUA,?
1. Department of Cardiology, Xuanwu Hospital Capital Medical University, Beijing, China; 2. Department of Geriatrics,Xuanwu Hospital Capital Medical University, Beijing, China
ABSTRACT OBJECTIVE To investigate the effects of Tongmai Yangxin Pill (TMYXP) combined with metoprolol tartrate or metoprolol alone for the treatment of premature ventricular complex (PVC) in patients with symptomatic frequent PVC. METHODS A total of 584 patients with symptomatic frequent PVC were randomly assigned (in a 1:1 ratio) into two groups:study group [n = 292, TMYXP (40 pills twice/day, orally) combined with metoprolol tartrate (25 mg twice/day, orally)]and control group [n = 292, metoprolol tartrate (25 mg twice/day, orally) plus placebo pill (40 pills twice/day, orally)]. The total treatment period was eight weeks. RESULTS After eight weeks of treatment, the total effective rate of reduction of PVC in the study group and the control group were 76.4% and 51.4%, respectively (P < 0.001). TMYXP combined with metoprolol tartrate demonstrated a significantly greater reduction of the frequency of PVCs compared with the metoprolol tartrate alone (?4537 times/24 h vs. ?3013 times/24 h, P < 0.001).The study group also showed a better result compared with the control group with respect to PVC related symptoms. In terms of New York Heart Association classification improvement, the total effective rates were 21.9% in the study group and 12.4% in the control group (P < 0.05). Both the study group and the control group exhibited improvements in echocardiographic indexes. Left ventricular ejection fraction was significantly improved in the study group compared with the control group (P < 0.05). There was no significant difference in the incidence of adverse events between the two groups. CONCLUSIONS Compared with metoprolol tartrate alone, TMYXP combined with metoprolol tartrate could more effectively reduce the frequency of PVC and alleviated PVC related symptoms, and improve cardiac function in patients with symptomatic PVC.
Premature ventricular complex (PVC) is a common type of arrhythmia, particularly in patients with structural heart diseases.It is often accompanied by symptoms such as palpitation, chest tightness, dizziness, and fatigue, some of the patients are highly symptomatic with impaired quality of life. Observational studies in general populations have revealed that frequent PVCs are associated with substantial elevations of risk for sudden cardiac death (SCD) and total cardiac death.[1]Several studies have demonstrated an association between frequent PVCs and potentially reversible cardiomyopathy.[2]Most clinical guidelines recommend betablockers as initial treatment for frequent PVCs with persistent symptoms, due to the tolerable safety profile and effectiveness in treating ventricular arrhythmia and reducing the risk of SCD.[2-4]The Cardiac Arrhythmia Suppression Trial study showed that sodium channel blockers can enhance post-myocardial infarction mortality.[5]Commonly used antiarrhythmic drugs have adverse effects (AEs) on liver,kidney, and thyroid function, most of these drugs are arrhythmogenic.[6,7]Catheter ablation can eliminate PVCs in 74%-100% of patients by selectively inhibiting the reentrant excitation pathway.[8]The application of an implantable cardioverter defibrillator greatly reduced the rate of sudden death in patients with high-risk ventricular tachycardia.[9]However,these invasive therapies have strict indications, in particular, catheter ablation of PVCs is recommended for a small number of patients who remain symptomatic despite conservative treatment, as well as for patients with very high PVC burdens associated with the decline in left ventricular systolic function.[2]In some hospitals within China, more than 10,000 daily PVCs is the indication for catheter ablation.[4]Medication therapy, therefore, is used for the majority of patients with symptomatic PVC. Consequently, there is an unmet medical need to explore other alternative treatment options with low toxicity and good efficacy profiles for frequent PVCs and could improve quality of life.
Although no terms are corresponding to “arrhythmia” in traditional Chinese medicine (TCM), the symptoms of arrhythmia are classified as “chest bi”and “palpitation”. Arrhythmia is presumably deficiency in origin and excess in superficiality; its origin is the deficiency of “Qi”, “blood”, “Yin” and“Yang”, whereas its superficiality is Qi stagnation,blood stasis, and retention of water. Thus, clinical manifestations are mostly a mixture of deficiency and excess. According to clinical practice and the published literature, TCM treatment of PVC has clear clinical efficacy and is widely used.[10-12]
Tongmai Yangxin Pill (TMYXP) is a Chinese patent medicine developed over many years of clinical practice. Previous research suggests that TMYXP exhibits significant overall efficacy and improvement of individual symptoms (e.g., chest tightness,palpitations, shortness of breath, fatigue, and dizziness),[12-14]however, the data of which are mainly from non-randomized controlled studies or small sample size studies, and the reported endpoints varied in different studies. Therefore, this multicenter, randomized, parallel-controlled clinical study was conducted to further investigate the effectiveness and safety of TMYXP combined with metoprolol tartrate compared with metoprolol tartrate alone for the treatment of PVC.
METHODS
Study Design and Population
This multicenter, randomized, double-blind, parallel-controlled clinical study was conducted in 21 sites in China. The study was approved by the Institutional Review Board of the lead site (Xuanwu Hospital Capital Medical University, Beijing, China; Clinical Trial Number: NCT05008250) and conducted under the ethical principles of the Declaration of Helsinki and all applicable laws and regulations. All the patients have signed informed consent to participate in this trial.
Inclusion criteria were as follows: (1) aged 18-75 years, male or female; (2) PVC with comorbidity of coronary heart disease (CHD) or without structural heart disease, the PVC frequency was 3000-30,000 times/24 h, with constant symptoms, especially affecting the quality of life; (3) PVC Lown grade II-IVA;(4) New York Heart Association (NYHA) grade I or II; (5) left ventricular ejection fraction (LVEF) ≥ 45%;and (6) written informed consent to participate in the trial. Exclusion criteria were as follows: (1) presence of bradyarrhythmia (< 50 beats/min), including sick sinus syndrome and atrioventricular block(second-degree or third-degree atrioventricular block); (2) presence of severe respiratory dysfunction or asthma; (3) poor peripheral circulation perfusion,severe peripheral vascular diseases; (4) presence of allergic constitution; (5) mental disorder; (6) ongoing beta-blocker treatment or contraindications to beta-blocker treatment; (7) pregnancy or lactation;(8) presence of persistent ventricular tachycardia,non-persistent ventricular tachycardia, and/or persistent atrial fibrillation; (9) presence of severe PVC requiring treatment with other antiarrhythmic treatment; (10) presence of drug-induced, electrolyte disorder induced, or acid-base imbalance induced arrhythmia; (11) presence of uncontrolled or severe hypertension (e.g., grade ≥ 3 hypertension); (12) presence of uncontrolled diabetes mellitus; (13) presence of alanine aminotransferase or aspartate aminotransferase level ≥ 1.5-fold above the upper limit of normal, blood urea nitrogen level ≥ 1.2-fold above the upper limit of normal, and/or serum creatinine above the upper limit of normal; and (14) participation in other clinical trials three months prior to the this study.
Randomization and Intervention
After three days of screening, patients were randomized to the study group or the control group in a 1:1 ratio. Stratified block randomization was used to allocate patients, SAS statistical software, version 9.3(SAS Institute, Inc., Cary, NC, USA) was used to generate a random number table. Patients and investigators remained masked to the randomization assignment for the duration of the study. Baseline data were collected during the screening period; three follow-up visits were conducted at week 2, week 4,and week 8, respectively. Vital signs, physical examination and AEs data were collected at each visit. Electrocardiography, echocardiography, and laboratory test data were collected at baseline and final visit.Twenty-four hour electrocardiography (Holter) was conducted at baseline, week 4 and week 8, respectively.
The study group received TMYXP (40 pills twice/day, orally) combine with metoprolol tartrate (25 mg twice/day, orally). The control group received metoprolol tartrate (25 mg twice/day, orally) plus placebo pill (40 pills twice/day, orally). Both groups received treatment for eight weeks. Patients with coronary disease took antiischemic therapy at the direction of investigators.
Efficacy Endpoints
The primary efficacy endpoint was the total effective rate of reduction of PVC after eight weeks of treatment. Effect on reduction of PVCs is defined as:(1) clinical control: PVC decreased by ≥ 90% compared with the baseline in Holter result; (2) marked effective: PVC decreased by 70%-90% compared with the baseline in Holter result; (3) effective: PMC decreased by 50%-69% compared with the baseline in Holter result; and (4) non-effective: PVC decreased by < 50% compared with the baseline in Holter result. The total effective rate was calculated as the composite of clinical control, marked effective and effective.
Secondary efficacy endpoints were as follows: (1)change in the number of PVCs per 24 h after treatment; and (2) change in total symptom score, the symptoms for this assessment included palpitation,chest discomfort, insomnia, dizziness, fatigue and breathlessness. Investigators finished PVC related symptom score assessments. The frequency and intensity of the symptoms were rated as none, mild, moderate, or severe, which corresponded to symptom scores of 0, 1, 2, and 3 points, respectively. Other secondary endpoints include changes in NYHA classification, and changes in echocardiography parameters (i.e., LVEF, left ventricular end diastolic dimension, E/A, cardiac index, cardiac output, and stroke volume).
Safety Assessment
Safety was investigated by examination of AEs,abnormal laboratory test results, and abnormal findings in physical examinations.
Statistical Analysis
This trial was designed based on the hypothesis that TMYXP combine with metoprolol tartrate therapy is superior to metoprolol tartrate alone in total effective rate of reduction of PVC after eight weeks of treatment. Based on published data, the total effective rate of reducing the number of PVCs/24 h at eight weeks in the control group was estimated to be 70%.Assuming a 10% increase in the total effective rate of reducing the number of PVCs/24 h in the study group and using an alpha-value of 0.05 and a beta-value of 0.2, the planned population of this study is 580 patients, with 290 patients in each group.
The full analysis set (FAS) was defined as all patients who received at least one dose of the study drug and had any efficacy data. These patients were the main population for efficacy analysis. The safety analysis set included all patients who received at least one dose of the study drug and had any safety data. All analyses were conducted using SAS statistical software, version 9.3 (SAS Institute, Inc., Cary, NC, USA).Statistical analysis was conducted using two-sided tests, aP-value < 0.05 was considered statistically significant.
For the total effective rate in reduction of PVCs, the Pearson’s chi-squared test was used. Cochran-Mantel-Haenszel test with adjustment for site effect was conducted to assess the between-group differences regarding the effective rate in NYHA classification.The change in the number of PVCs was compared between the two groups by the Mann-WhitneyUtest.To examine changes in total symptom score and echocardiography parameters, an analysis of variance was used.
RESULTS
Patient Characteristics
Patient disposition is summarized in Figure 1. A total of 600 patients were originally screened for eligibility for the study, 16 patients of whom did not meet the inclusion criteria and were therefore not evaluated further, leaving 584 patients (292 patients in the study group and 292 patients in the control group) recruited for both of the FAS and safety analysis set. The mean age in the study group was 56 years (range: 18-76 years), and 56.8 years (range:18-77 years) in the control group, while the median age in the study group was 58 years, and 59 years in the control group. There were no significant differences between the two groups in the baseline characteristics (Table 1).
Efficacy Results
Primary endpoints: after eight weeks of treatment,in the FAS analysis, the total effective rate of the reduction of PVCs was significantly higher in the study group (P< 0.001, Table 2). In the study group, 92 patients achieved “clinical control”, 71 patients achieved “marked effective”, and 60 patients achieved“effective”. A total of 223 patients are regarded as effective and the total effective rate is 76.4%. In the control group, only 150 patients are regarded as effective, with a total effective rate of 51.4%.
Secondary endpoints: after eight weeks of treatment, in the FAS analysis, the numbers of PVCs/24 h decreased by ?4537 in the study group and ?3013 in the control group (P< 0.001, Table 3). A significant decrease in the numbers of PVCs/24 h has been shown in the study group at week 4. Results of other secondary endpoints are summarized in Table 4. Compared with the control group, the total symptom score was significantly improved in the study group at week 4 and week 8 (bothP< 0.001). At baseline, the total symptom score was 5.75 in the study group, and 5.48 in the control group. At week 4, the symptom score was decreased by ?2.76 in the study group, whereas it decreased by ?1.79 in the control group. At week 8,the difference between groups was more significant(?4.33vs.?2.86). The total effective rate for NYHA classification was 21.9% in the study group and 12.4%in the control group (P= 0.004). After eight weeks of treatment, LVEF increased by 1.2% in the study group and 0.21% in the control group, showing a significant difference between the two groups (P= 0.040).There was an increase in left ventricular end diastolic dimension, cardiac index, cardiac output, and stroke volume in both groups, without significant difference between the two groups (P= 0.828,P= 0.912,P= 0.733, andP= 0.940, respectively).
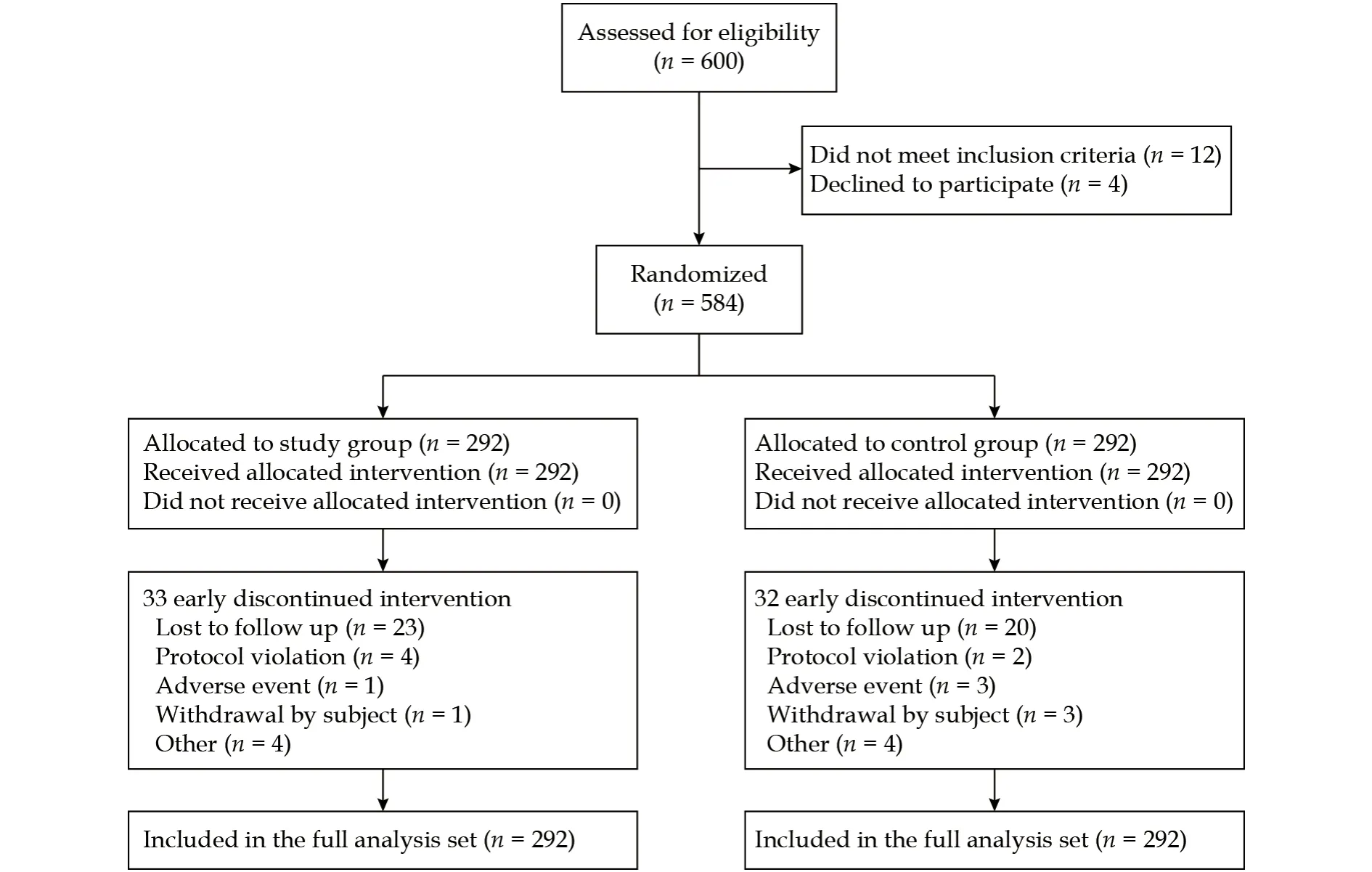
Figure 1 Flow chart of patient recruitment and study completion.
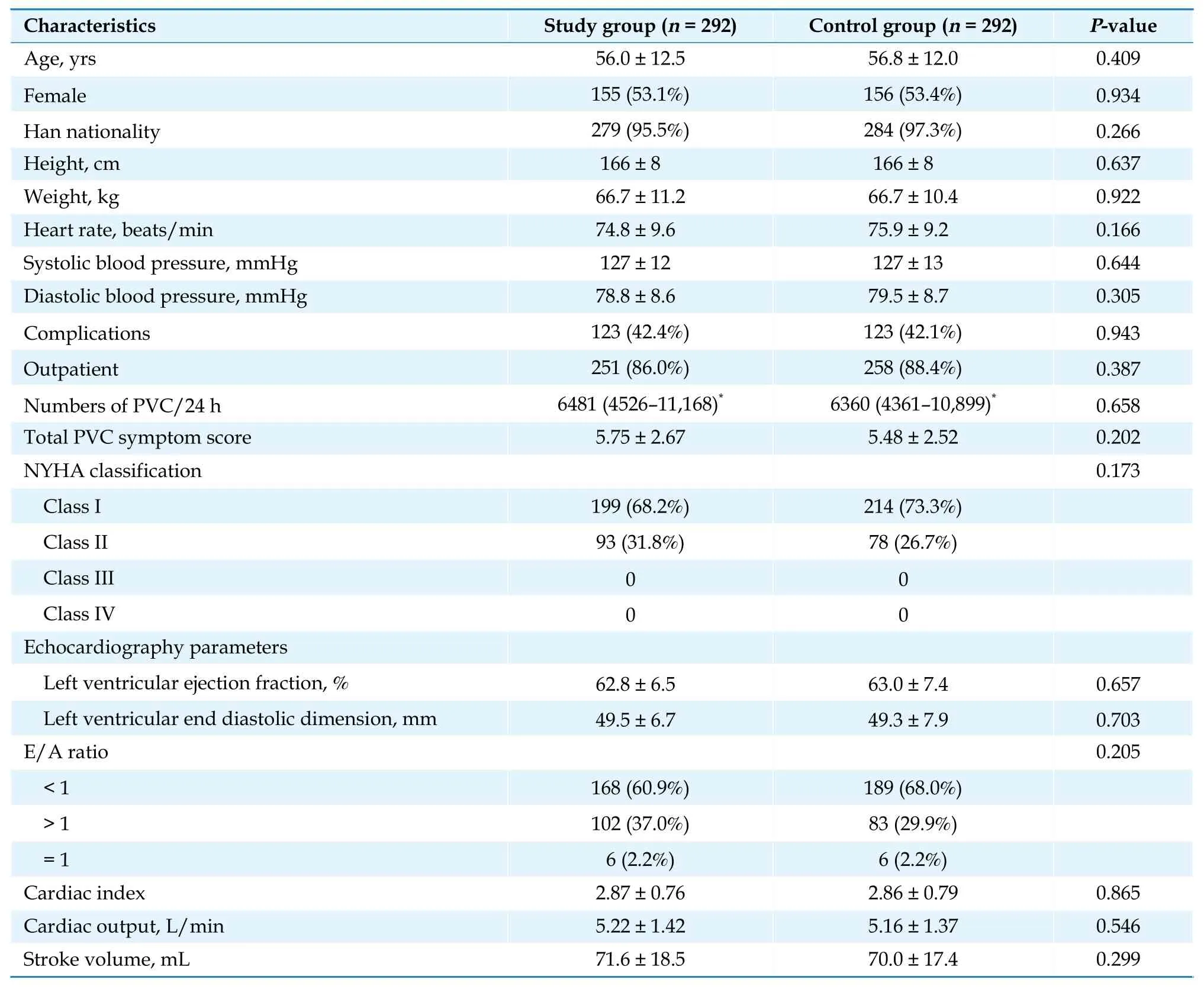
Table 1 Patient demographics and baseline characteristics: full analysis set.
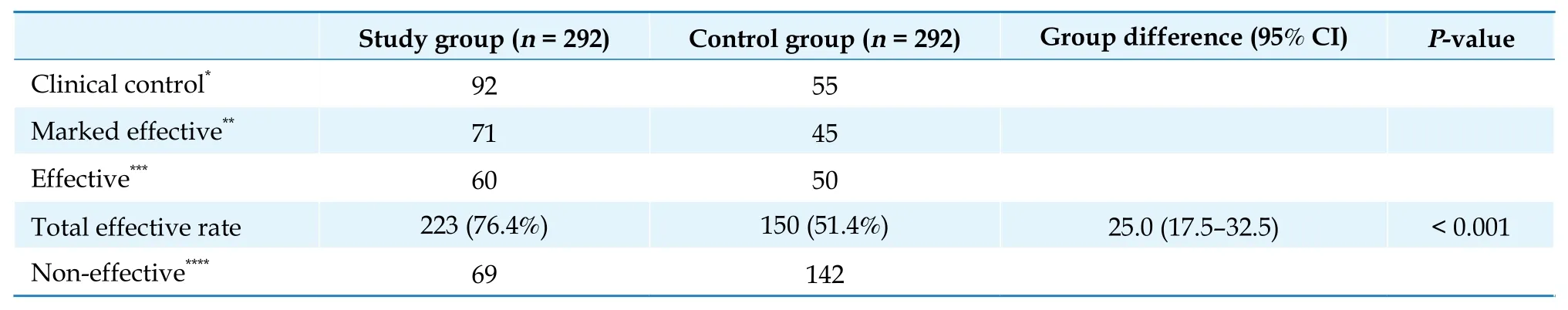
Table 2 Effective rate of improvement in 24 h premature ventricular complex: full analysis set.
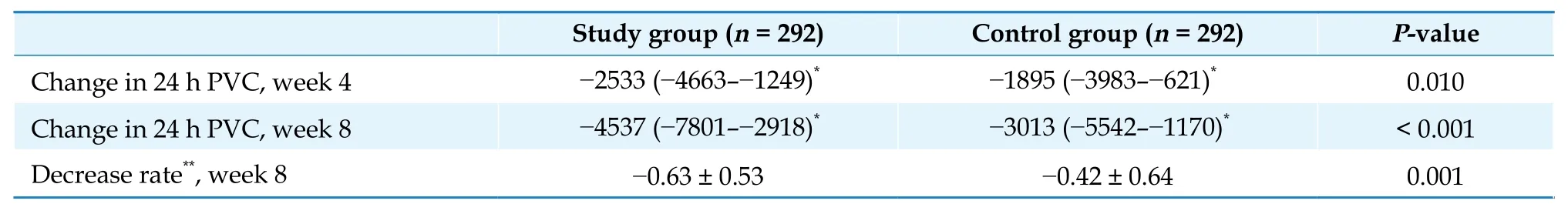
Table 3 Improvement of 24 h PVC: full analysis set.
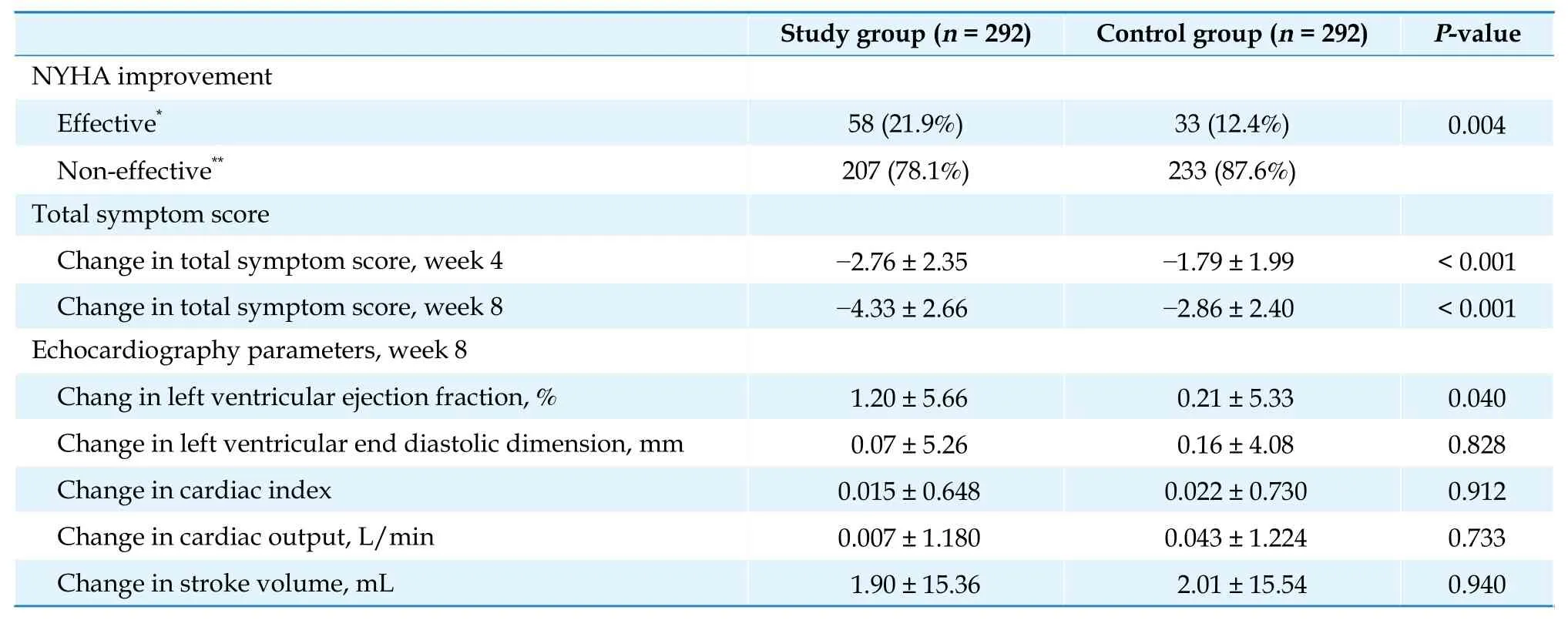
Table 4 Results of other secondary endpoints: full analysis set.
Safety Results
A total of 76 AEs were reported, 37 AEs of which occurred in the study group (AE incidence of 12.7%);three of the 37 AEs were regarded as drug-related(1.0%). 39 AEs occurred in the control group (AE incidence of 13.4%); seven of the 39 AEs were regarded as drug-related (2.4%). There was no statistical significance in the incidences of AEs or drug-related AEs between groups (bothP> 0.05). No serious AEs occurred in either group. Drug-related AEs in the study group included dizziness, headache, nausea and vomiting; drug-related AEs in the control group included headache, nausea, diarrhea and stomachache.
DISCUSSION
Antiarrhythmic medications are often categorized by the Vaughan Williams 4-level schema, in this system, metoprolol tartrate (a heart-selective betablocker) is a class II antiarrhythmic medication that decreases the ventricular rate of supraventricular tachyarrhythmias by inhibition of atrioventricular conduction. Beta-blockers are often first-line antiarrhythmic therapy and are recommended for the treatment of PVC, because of the excellent safety profile and effectiveness in treating ventricular arrhythmia and reducing the risk of SCD.[3,15]However, the efficacy of beta-blockers is limited, such that only 10%-15% of patients achieve more than 90% PVC inhibition with this treatment.[16,17]
TMYXP is a Chinese patent medicine developed over many years of clinical practice. The ingredients of TMYXP include Radix Rehmanniae, Caulis Spatholobi, Polygonum Multiflorum, Colla Corii Asini,Ophiopogonis, Tortoise Shell (vinegar), Radix Codonopsis, Cassia Twig, Jujube, Schisandra Fruit, and Licorice Root. It has the effect of invigorating Qi,nourishing Yin, dredging the pulse, and relieving pain. It is used in the treatment of Qi and Yin deficiency syndromes caused by CHD, angina pectoris,and arrhythmia. The results of previous research suggest a good overall efficacy of TMYXP in treating PVC, as well as relief of symptoms (e.g., chest tightness, palpitations, shortness of breath, fatigue,dizziness, and dry mouth).[12-14]Basic science studies have revealed 40 bioactive components, some of which inhibit epithelial-mesenchymal transition and inflammation.[18-20]Another basic science analysis showed that TMYXP could inhibit calcium overload in myocardial cells, thereby reducing the incidence of myocardial apoptosis.[21]
The efficacy results showed that the primary endpoint was achieved. After eight weeks of treatment,the total effective rate of TMYXP combine with metoprolol tartrate in reducing the number of PVCs/24 h was significantly higher than using metoprolol tartrate alone; the difference between the study group and control group in terms of decrease in the total number of PVC is growing over time, such that there were significant differences at week 4 and week 8.The total effective rates were 76.4% and 51.4% in the study group and the control group, respectively, which are relatively lower than those reported in prior studies.[12-14]It may be attributed to the presence of more severe PVC in the patients of this study, however,all studies indicate that TMYXP enhances the efficacy of treatment for PVC.
For other secondary efficacy endpoints, TMYXP combined with metoprolol tartrate showed better efficacy than metoprolol tartrate alone in terms of improvements in symptom score, which is consistent with previous findings. Several TCMs were proved to be effective in reducing PVC-related symptoms including palpitation, chest discomfort, insomnia and fatigue, symptom alleviation, and could improve the quality of life significantly.[22]There was also a significant improvement in NYHA classification. Echocardiographic parameters were improved in both groups compared with baseline, improvement of LVEF was better in the study group than in the control group (P< 0.05). Patients with chronic heart failure often have ventricular arrhythmias. Furthermore, PVC may cause reduction in cardiac output. Thus, reducing PVC may lead to improvement of cardiac function; alternatively, TMYXP may inhibit calcium overload in myocardial cells, which is cardioprotective and might reduce the onset of ventricular arrhythmias. To the best of our knowledge,this is the first study to show TMYXP combined with metoprolol tartrate can improve cardiac function in patients with PVC.
The safety result suggested a tolerable safety profile of TMYXP combined with metoprolol tartrate.Incidences of AEs and drug-related AEs were similar between groups in this study. The AEs were mild;no serious AEs occurred throughout the study. Package inserts for TMYXP did not state specific adverse reactions associated with TMYXP treatment. The drug-related AEs in the present study were similar to the package insert that listed common AEs associated with metoprolol tartrate treatment.
LIMITATIONS
This study was limited to eight weeks of followup, which may not be sufficient to evaluate the longterm efficacy and safety of TMYXP combined with metoprolol tartrate for the treatment of symptomatic frequent PVC. In addition, this study only enrolled PVC patients with CHD or without structural heart diseases, PVC with other structural heart diseases such as primary cardiomyopathy, or valvular heart disease, should be included in future clinical trials.
CONCLUSIONS
This multicenter, randomized, parallel-controlled clinical study shows that TMYXP combined with metoprolol tartrate has a better result as compared with metoprolol tartrate alone for the treatment of PVC,as indicated by a higher total effective rate of improvement in PVCs and better improvement in the number of PVCs/24 h, as well as a better symptom relief.The underlying mechanism of antiarrhythmia needs to be further explored. The study also demonstrated the potential of TMYXP for improving cardiac function in patients with PVC, which needs to be further investigated in future studies.
ACKNOWLEDGMENTS
This study was supported by the Tianjin Lerentang Pharmaceutical Factory. All authors had no conflicts of interest to disclose.
 Journal of Geriatric Cardiology2022年4期
Journal of Geriatric Cardiology2022年4期
- Journal of Geriatric Cardiology的其它文章
- Rotor hypothesis in the time chain of atrial fibrillation
- 3D vena contracta area in degenerative mitral regurgitation:cross-platform comparison in a single patient
- New-onset heart failure masking a massive retroperitoneal liposarcoma
- Invasive versus non-invasive hemodynamic monitoring of heart failure patients and their outcomes
- Implication of a novel truncating mutation in titin as a cause of autosomal dominant left ventricular noncompaction
- Trends and sex differences in atrial fibrillation hospitalization and catheter ablation at tertiary hospitals in China from 2013 to 2016
