lnsights on the possibility of SARS-CoV-2 transmission through the eyes
Rui Zhu, Zi-Yan Yu, Lin Han
1Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Shenyang 110005, Liaoning Province, China
2Eye Hospital of China Medical University, Shenyang 110005,Liaoning Province, China
3Key Lens Research Laboratory of Liaoning Province,Shenyang 110005, Liaoning Province, China
4China Medical University, Shenyang 110122, Liaoning Province, China
Abstract
● KEYWORDS: coronavirus; SARS-CoV-2; ocular;conjunctivitis
INTRODUCTION
Human coronaviruses (HCoV) belongs to single-chain RNA viruses, existing six other kinds of coronaviruses to infect the human body: HCoV-OC43, HCoV-229E, HCoVNL63 and HCoV-HKU1, usually account for common colds;severe acute respiratory syndrome coronavirus (SARS-CoV)and middle east respiratory syndrome coronavirus (MERSCoV) take charge of outbreaks of pandemic originated in 2002 and 2015, respectively—with mortality rates of 10%for SARS-CoV, 37% for MERS-CoV[1-4]. The SARS-CoV-2 currently circulating worldwide belongs to the third highly pathogenic coronavirus. SARS-CoV-2 based on RNA structure inheres higher rates of mutation than DNA viruses. Most of the mutation sites fail to change biological characteristics of the coronavirus, while a few variants whose genes of encoding Spike protein mutate can produce changes in critical amino acids[5]. The occurrence of variants made themselves more easily transmitted, more pathogenic, and less sensitive to neutralizing antibodies in immune reactions[6-7]. For example,SARS-CoV-2 Delta VOC (B.1.617.2), discovered firstly in October 2020, and SARS-CoV-2 variant Omicron (B.1.1.529),discovered firstly in November 2021, have aroused a rebound of the epidemic[5,8]. The new infections driven by Omicron have risen sharply since early December and the highly contagious Omicron variant even has overtaken Delta as the predominant lineage in South Africa[9].
More than 400 million people have been infected and 5.78 million passed away according to data of the World Health Organization, as of early February 2022. Droplets, close contact and aerosols are three main routes of transmission of SARS-CoV-2[10]. However, a medical expert was infected by 2019-nCoV during the inspection in Wuhan Fever Clinic.He wore an N95 mask but did not wear anything to protect his eyes, suggesting that 2019-nCoV transmission through the ocular surface must not be ignored[11]. In a case report,a new type of atypical keratitis is a clinical manifestation of COVID-19, which could appear 3d earlier than common symptoms of COVID-19[12]. It is indicated that the cornea and conjunctiva are one of the transmission routes of COVID-19. From anatomy, close connection of the eye and the respiratory systemvianasolacrimal duct creates a facility for virus invasion[13]. The identification of the cellular receptors employed by coronavirus gives insight into possible infection mechanism. Ocular tropism of other viruses expedites to figure coronavirus-eye interaction out. Together, these findings all increase our speculations about what role the eye plays in the spread of the virus. Further, these also reveal the importance and necessity of safe care operations in close ophthalmology practices. Hence, the correlation between coronavirus and the ocular surface should be expounded at great length on grounds of prevalence of coronavirus.
In this review we analyze coronavirus-eye correlation and discuss ocular tropism of other viruses so as to determine risk of ocular transmission. Furthermore, we roll out preventions and care measures for ophthalmologists and nurses in an effort of reducing the prevalence of coronavirus, especially as performing eye operations.
EVIDENCE ON CORONAVIRUS AND EYE DISEASE
Possible Mechanisms of Eye as a Transmission Route The eye may take effect as a coronavirus replication site and a potential gateway for virus entering the respiratory system.Seven human coronaviruses all depend on their surface spikelike S-glycoprotein, composed of two subunits, to hook up to surface receptors of host cells and then access the cells mediating subsequent processes of cell membrane fusion[14-15].While coronaviruses of different kinds recognize diverse cell surface receptors, some receptors identified by coronavirus are confirmed to be distributed in the eye. It is indicated the eye has a molecular basis of virus invasion through the expression of receptors.
Both SARS-CoV and SARS-CoV-2 make use of the receptor—human angiotensin converting enzyme 2 (ACE2) to identify host cells and then prime S-glycoprotein with help of the cellular serine protease TMPRSS2[16]. Interestingly, the SARSCoV-2’s spike protein possesses more tenfold higher stickness to ACE2 than SARS-CoV’s[17]. Recent studies report CD147-spike protein connection is a newfound route for SARSCoV-2 to infect host cells; CD147 is an alternative receptor especially in types of ACE2-deficient cell[18]. Significantly,immunohistochemical analysis reveals the expression of ACE2 in conjunctiva, limbus, and cornea beyond respiration tract while CD147 and TMPRSS2 are expressed at intermediate levels in both conjunctiva and cornea[19-20]. The presence of permissive receptors can go a long way toward the tropism of viruses to ocular tissue[21]. Host cell entry of MERS-CoV resorts to the receptor-dipeptidyl peptidase 4 (DPP4), also known as CD26[22]. Outside of DPP4, another leading receptor attached to respiratory epithelial cells is sialic acid: MERSCoV displays a more preference for α2,3-linked sialic acids rather than α2,6-linked sialic acids[23]. Corneal conjunctival epithelial cells are affluent in α2,3-linked sialic acids[24];lacrimal sac and nasolacrimal duct express both kinds of saliva acid[25]. However, there is no literature to state that DPP4 can be expressed in the human eye so far. As a longitudinal study conducted in 9 naturally infected dromedary camels, MERSCoV nucleic acids were detected in conjunctival swabs of infected animals at 1 case (11%), 3 cases (33%), and 2 cases(22%) during the three-time points, respectively and some of them showed mild conjunctivitis. Nevertheless, ocular manifestations in human with MERS-CoV remain rare, with only 2% (6/261) of cases reported having conjunctivitis in a retrospective study conducted in the Makkah Region of Saudi Arabia[26-27]. This situation suggests DPP4 or other potential receptors may play a decisive influence on virus tropism.Several receptors of other coronaviruses are summarized in Table 1. While shared receptors distribution can be in favor of ocular tropism of virus, the mechanism of determining virus tropism remains a mystery and a more detailed explication is necessary.
Assuming coronaviruses can attack the eye, following how do viruses get into respiratory tract? Anatomical connections may answer to the course of virus transfer from ocular surface to other tissues. Nasolacrimal duct establishes a bridge to connect eye tissue with respiratory tract[28]. Tear fluid on the surface of the eyeball are collected into the lacrimal sac and then flow through the nasolacrimal duct into the nasal cavity and subsequently into the respiratory tract[13]. If the tear fluid contains enough viruses arousing pathogenic infection, a distant respiratory illness would take place. The processes of how coronaviruses invade respiratory system are illustrated in Figure 1. An animal experiment stands up for the hypothesis of ocular route. Rhesus macaques were inoculated with 1×106TCID50 of 2019-nCOV by means of conjunctival, intragastric,and intratracheal injection. While caused interstitial pneumonia is mild following 2019-nCOV inoculation by the conjunctival route, the conjunctivally injected animal is detected a higher viral load concentrated in the nasolacrimal system especially conjunctiva, lacrimal gland, nasal cavity and throatviaautopsies than the animal with intratracheal injection[29]. It is a pity that the experiment only used a small amount and a single test dose with great limitations. The conjunctival route is notwithstanding required profound studies to offer definitive evidence for ophthalmologists and the public.
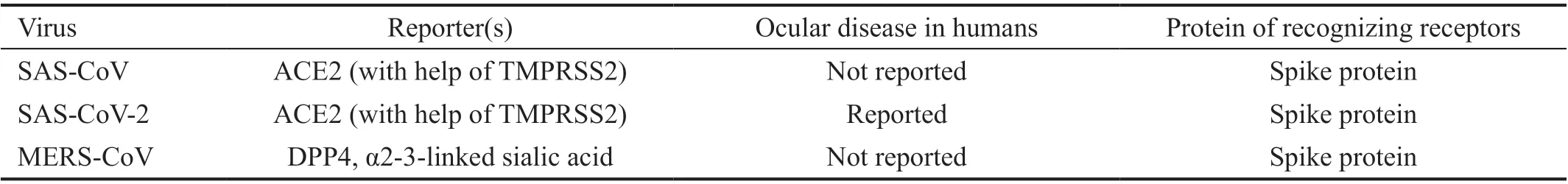
Table 1 Summary of reporters and ocular disease of coronaviruses
In addition to the passage of nasolacrimal system at the anatomical level, a discovery of 2019-nCOV in human retina takes the route of hematogenous metastasis into consideration[30]. One possible passageway is summarized as follows: initially SARS-CoV-2 infects iris and conjunctival cells via ACE2 and CD147 receptors to reach blood capillaries and choroid plexus[31]; next, the virus encounters the obstruction of blood-retinal barrier (BRB) with expressing both ACE2 and CD147 in retinal pigment epithelium (RPE)and blood vessel endothelial cells; what’s more, CD147 intervenes collapse of neurovascular barriers when the host is under inflammatory conditions[32]; ultimately, with the help of CD147, the virus can cross the BRB and enter blood to infect extraocular areas. Albeit in theory, blood transfer of SARSCoV-2 from ocular surface is possible, but this assumption requires more experimental evidence to certify.
Accumulated Clinical Cases Were Reported Ocular Symptoms of COVID-19At the beginning of the outbreak of COVID-19, a number of ophthalmologists were reported to have died of the virus infection including Dr. Li Wenliang,who was known as one of the earliest doctors to discover the virus[33]. With increasing attention of clinicians about the link between ophthalmology and coronavirus, multiple clinical cases of COVID-19 related to eye symptoms have been reported. A Meta-analysis illustrated the most prevalent ocular manifestation was conjunctivitis among SARS-CoV-2 patients[34]. Based on the systematic review of Jinet al[35],hospital-based cross-sectional studies showed the range from 0 to 31.6% of COVID-19 patients have conjunctivitis with 75%reported rates less than 4.0% and eye symptoms were reported ranging from 5.0% to 26.8%. In addition to SARS-CoV-2,HCoV-NL63 has also been observed with eye symptoms. In a retrospective study of 300 hospitalized children, conjunctivitis occupied a share of 16.7% (3 out of 18) in patients diagnosed with HCoV-NL63 from November 2002 to April 2003[36].
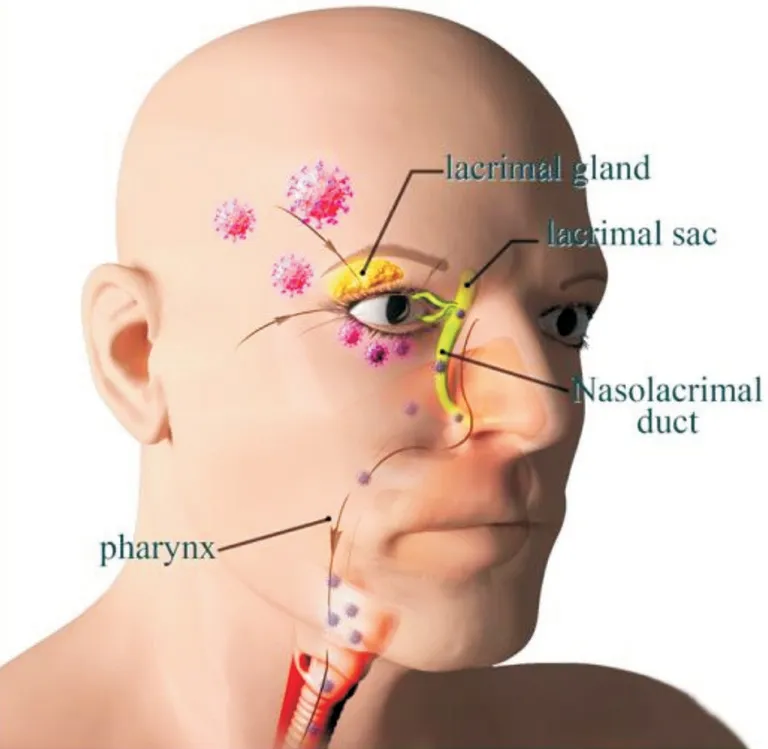
Figure 1 Illustration of entering the respiratory system through the eyes Coronaviruses first attack the eyes and then follow the flow of tears into the lacrimal sac. Next they flow through the nasolacrimal duct and thence to the pharynx and end up in the lungs.
If SARS-CoV-2 can invade the body through the route of ocular transmission, it is mostly possible that the initial symptom was found in eye[37]. Ozturker[38]reported that a nurse, who suffered from conjunctivitis as the first and sole sign, resulted from inevitable eye exposure in the Emergency Department and subsequently was confirmed as COVID-19.There is another case reporting an infected person of SARSCoV-2 with keratoconjunctivitis as the main symptom[39].In this case, a retrospective test manifested the conjunctival swabs initially collected was weakly positive for SARSCoV-2. But the first patient had negative RT-PCR results for ocular samples. It is worth noting both two patients were accompanied by symptoms of redness of the eye, watery or mucous discharge of the eye and photophobia. These findings provide further evidence of ocular transmission.
Detection of Coronavirus in Conjunctival Secretions and TearsConjunctival swab is a kind of technique to collect conjunctival secretions and tear samples. Series of cases reported detection of coronavirus by means of conjunctival swabs suggesting reproductions of viruses in conjunctival secretions and tears. Loonet al[40]collected conjunctiva secretions from eight patients with SARS-CoV and detected viral RNA in three patients: two of them were elder male with multiple medical conditions while the third was a young female healthcare laborer. In spite of numerous investigations subscribe to a connection between ocular symptoms and coronavirus, the rate of positives by RT-PCR remains low.According to a systematic review, the range of positive conjunctival swab results was about 1%-5% in patients diagnosed with COVID-19 accompanied with ophthalmic symptoms[41]. There may be many reasons for this contrast between low positive detection rates and relatively high ocular manifestations. First, the window period for the virus to fall off from ocular tissues might be too short to detect. And which stage of COVID-19 does it occur in is not definitively certain[41]. Then, the amount of virus load in the ocular tissues also play an important role. The lack of standardized handling or testing protocol for collected samples may give rise to false negative rates eventually[42-43]. Xieet al[44]reported that ocular surface swabs for SARS-CoV-2 were strongly positive from 2 of 33 patients without any ocular manifestations. Despite the low positive rates, there is no denying that coronavirus RNA exists in conjunctival secretions and tear samples, which implies that this virus might be spread through conjunctival contact. In conclusion, ocular involvement of coronavirus cannot be neglected during the COVID-19 pandemic.
Ocular Tropism of Other VirusesDespite different incidences of ocular diseases in wide varieties of respiratory viruses, many common characters of the interaction between pathogens and the eye can help us shed light on the link between coronavirus and the eye. Adenoviruses, typically related to respiratory disease, can be observed to produce severe eye infections determined by specific strains such as serotypes 8, 19, and 37 of species D adenoviruses[45]. A large proportion of species D adenoviruses can cause light conjunctivitis, manifesting follicular changes[46]. Serotype 37 is one of the common pathogens that cause epidemic keratoconjunctivitis (EKC),and researchers can often detect the pathogens from eye swabs of patients with EKC[47]. The tropism of adenoviruses for ocular tissue corresponds with its property to bind to sialic acid existing on the corneal and conjunctival epithelium[48].Remarkably, species D adenoviruses have been demonstrated greater patience and resistance in the face of ocular cytokines with defensibility than other species[49]. Followed by adenovirus replications in the eyes, a deal of cytokines and NF-кB would take part in initiation and progression of inflammation[50]. On all accounts, vital receptors and cytokines facilitate adenoviruses to spreadviathe eye.
Influenza A virus is a common influenza virus prone to mutation and some strains causing acute respiratory infections have set off more than one pandemic in the world[51].Typical symptoms of influenza are generally concerned with respiratory system. Nevertheless, accompanied ocular manifestations have caught public attention especially subtype H7 viruses. Many recorded clinical cases indicate subtype H7 viruses increase the incidence of conjunctivitis and high infectiousness of influenza may be attributed to involvement of the eye during transmissions[52]. The eye can be engaged in direct and indirect contact. For example, hands coming into contact with conjunctival secretions and then exposure to mouth, nose, eye mucosa can give rise to an infection to a large extent. Besides, viruses droplets excreted by patients can directly invade conjunctiva and replicate in ocular surface[53].A fewin vitroandin vivoresearches have been performed to notarize the permission of ocular cells to influenza viruses.Results reveal that human corneal and conjunctival epithelial cells could be available for rapid propagation of influenza H7 subtype as well as do some cells that positioned inside the eye can also[54]. Infection emerged in the upper respiratory tract following ocular inoculation in ferrets with avian influenza viruses[55]. The animal model testified transfer capacity of the viruses from ocular tissue to respiration tract. Consequently,the special influence of eye in the spread of influenza viruses can-not be ignored.
Coxsackievirus A24 variant (CVA24v) and enterovirus type 70 (EV70) are answerable to a great number of occurrence of acute hemorrhagic conjunctivitis (AHC)[56]. Clinical features of AHC are acute attack of eye pain, swelling of the eyelids,foreign body sensations, as well as even wide subconjunctival hemorrhage[57]. CVA24v depends on intercellular adhesion molecule-1 (ICAM-1) as the primary receptor and Sialic Acid is another significant receptor that link up corneal and conjunctival with the pathogen[58]. Further studies point out that there exists a single capsid substitution—Tyr250 strengthening viruses’ combination with Sialic Acid in pandemic CV-A24v strains[59]. The specialty of CVA24v suggests virus tropism may be subject to potential and undiscovered receptors.
PRECAUTIONS FOR OPHTHALMOLOGISTS AND NURSES DURING SARS-COV-2 EPIDEMIC
Susceptibility of SARS-CoV-2 to Ophthalmologists and NursesEven though the route of ocular transmission is not yet proven, characters of ophthalmic practices put ophthalmologists and nurses at high risk of viral infection.In outpatient clinic, some examination procedures inevitably require close contact with patients. For example, when using direct ophthalmoscope, the distance between the mouth and nose of the doctor and patient is less than 20 cm, which is conducive to spreading virus by respiratory droplets. Hence binocular indirect ophthalmoscope is recommended to replace direct ophthalmoscope temporarily owing to the longer doctorpatient distance of the former[14].
Conjunctival sac irrigation is a routine preoperative operation of ophthalmic surgery, but the irrigation process is prone to aerosols, which causes a safety hazard. Similarly, during surgical procedures, the splash of intraocular irrigation fluids and frequent exposure to tears and ocular discharge may increase risk of infection[60]. So it is necessary for medical staffin ophthalmology working on the front lines to carry out strict precautions and nursing care to avoid cross-infection.
Recommended Precautions and Nursing CareThe basic strategies that are recommended for ophthalmologists and nurses to overcome the COVID-19 challenge include appropriate personal protective equipment (PPE); limiting overcrowding in medical facilities; detailed travel, occupation,contact and cluster screening; safe hand hygiene practices and so on[61]. Ophthalmologists and nurses should keep highly wary of these patients with respiratory symptoms such as cough, chest distress and shortness of breath and take extra preventive measures such as goggles, face shield, gloves,N-95 mask and protection suit[62]. A proper disinfection protocol for all potentially contaminated equipment such as slit-lamp biomicroscope and lenses is also essential. Large respiratory shields mounted on slit lamps are currently being utilized in many ophthalmic institutes because they can benefit for minimizing droplet exposure. At the same time strict disinfection should be carried out after use by different patients to prevent contamination of the shields and infection between patients[14]. In addition, considering the complexity of infectious factors, nursing safety in the ophthalmic operating room should be of particular concern.
When preparing for surgery, it is important to plan the tasks to be performed, the minimum necessary elements inside the operating room and the assignment of roles[63]. Strictly control the number of people in the operating room and reduce the flow of personnel as much as possible to prevent crossinfection. Streamline the placement of items in the operating room and place only the necessary instruments and equipment.Increase the cleaning and disinfection of microscope objectives to prevent contamination caused by splattering fluid at the end of each procedure[64]. When receiving intravitreal injections,patients are required to wear standard surgical masks for protection. In an emergency, if the patient does not have a negative nucleic acid certificate, the doctor should wear an isolation suit to perform the operation. Furthermore, for other ophthalmic procedures such as eye trauma suturing, local eye surgery and so on, appropriate personal protective equipment is necessary for both doctors and patients. The safety of nursing care in the operating room is closely related to the incidence of infection. Healthcare workers should raise awareness of infection prevention and take personalized nursing measures based on the patient’s condition.
CONCLUSIONS
Overall, ocular distributions of receptors identified by coronavirus cement a foundation for viruses entrance and grant viruses rights to replicate in eyes. What’s more, nasolacrimal system and hematogenous metastasis contribute to virus transformation from the ocular surface to other locations to bring about infection of a larger scale. Although conjunctivitis is not a common symptom of coronaviruses, the appearance of virus RNA in conjunctival swab reinforces reliable proofs for ocular route of coronavirus transmission. However, more clear evidence and further researches are required to elucidate the role of the eye in coronavirus infection. Based on current evidence, ophthalmologists and nurses on the front lines must take full account of the possibility of coronavirus transmission through the ocular route and take strict protection and adequate precautions in close eye practices to assist reducing further prevalence of SARS-CoV-2.
METHOD OF LITERATURE SEARCH
A detailed search was done on PubMed with a combination of the search terms not limited to “Coronavirus”, “SARSCoV-2”, “conjunctivitis”, “eye”, “ophthalmology”, “ocular”.Articles were restricted to English language. Article type was not limited.
ACKNOWLEDGEMENTS
Foundation:Supported by National Natural Science Foundation of China (No.82000877).
Conflicts of Interest:Zhu R,None;Yu ZY,None;Han L,None.
 International Journal of Ophthalmology2022年11期
International Journal of Ophthalmology2022年11期
- International Journal of Ophthalmology的其它文章
- Paracentral acute middle maculopathy after diagnostic cerebral angiography in a patient with a contralateral carotid-cavernous fistula
- Optical coherent tomography angiography observation of macular subinternal limiting membrane hemorrhage in CO poisoning: a case report
- Choroidal folds associated with carotid cavernous fistula:a case report
- Role of the ultra-wide-field imaging system in the diagnosis of pigmented paravenous chorioretinal atrophy
- A rare combination of late-onset post-LASlK keratectasia after early-onset central toxic keratopathy
- Gastrointestinal microbiome and primary Sj?gren’s syndrome: a review of the literature and conclusions
