Choroidal folds associated with carotid cavernous fistula:a case report
Yasutsugu Akasaki, Takenori Inomata,3,4, Jaemyoung Sung, Megumi Ito, Hiroaki Kobayashi,Ryosuke Kuwana,6, Hidenori Oishi, Akira Murakami
1Department of Ophthalmology, Juntendo University Graduate School of Medicine, 3-1-3 Hongo, Bunkyo-ku, Tokyo 113-0033,Japan
2Department of Digital Medicine, Juntendo University Graduate School of Medicine, 3-1-3 Hongo, Bunkyo-ku,Tokyo 113-0033, Japan
3Department of Strategic Operating Room Management and Improvement, Juntendo University Graduate School of Medicine, 3-1-3 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
4Department of Hospital Administration, Juntendo University Graduate School of Medicine, 3-1-3 Hongo, Bunkyo-ku,Tokyo 113-0033, Japan
5Morsani College of Medicine, University of South Florida,560 Channelside Dr, Tampa, FL 33602, USA
6Kuwana Ophthalmology Neurosurgery Clinic, 27-1 Kumaiden,Shimizu-cho, Sunto-gun, Shizuoka 411-0911, Japan
7Department of Neuroendovascular Therapy, Juntendo University Faculty of Medicine, 3-1-3 Hongo, Bunkyo-ku,Tokyo 113-0033, Japan
8Department of Neurosurgery, Juntendo University Faculty of Medicine, 3-1-3 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
Dear Editor,
I am Yasutsugu Akasaki, from the Department of Ophthalmology, Juntendo University Hospital. I am writing this letter to present a case of choroidal folds associated with carotid cavernous fistula (CCFs).
CCFs represent various forms of abnormal communication between the cavernous sinus and carotid arterial systems,and may be idiopathic, traumatic, or iatrogenic[1]. CCFs may be classified into high- or low-flow types depending on the hemodynamics surrounding the abnormal shunt. CCFs are also classified into Barrow type A, B, C, or D according to their anatomy. Barrow type A CCFs are the most common and exhibit a direct shunt connecting the internal carotid artery(ICA) to the fistula, which commonly results in high-flow lesions. Barrow type B, C, and D CCFs are indirect shunts arising from the meningeal branches of either the ICA or external carotid artery (ECA), which usually result in low-flow lesions. Barrow type B, C, and D CCFs involve the meningeal branches of the ICA, ECA, and both branches of the ICA and ECA, respectively[2-4]. Ocular manifestations of idiopathic CCF include hyperemia, exophthalmos, diplopia, increased intraocular pressure, dilation of the superior ocular veins,extraocular muscle thickening, congestive retinopathy, central retinal vein occlusion, and choroidal dissection[5-12].
Choroidal folds (CFs) are often observed as a subretinal lines, grooves, or striae. The lines are typically parallel to each other in a horizontal fashion, but these may be vertical,oblique. In addition, since mild CFs are often difficult to detect by ophthalmoscopy or fundus photography, further examinations are required using optical coherence tomography(OCT) or fluorescence fundus angiography. Numerous pathologies have been associated with the development of CFs,including hypotony, choroidal tumors/detachment, Graves’ophthalmopathy, orbital masses, inflammation, infection, optic nerve disorders, posterior scleritis, uveitis, hypermetropia, and CCF[13-16].
Despite reports on and the establishment of an association between CCF and CFs[17-18], the clinical course and management outcomes of CCF with CFs have not yet been investigated.Herein, we report a case of idiopathic CCF with CFs and describe the clinical course of the CFs after coil embolization of the fistula. The study was approved by the Ethics Committee of Juntendo University Hospital (Approval number,JHS 21-0027) and adheres to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
CASE PRESENTATION
A 77-year-old woman complained of redness and eyelid swelling of her left eye in 2014 and underwent an examination at the hospital.
She had a history of incomplete Beh?et disease (oral ulcers,genital ulcers, and uveitis) in 2009, which had been treated with colchicine, and a history of temporary diplopia caused by left oculomotor paralysis from stenosis of the left middle cerebral artery in 2012, which had been treated with aspirin from 2012 to 2013. In 2013, she complained of redness and left eyelid swelling, and magnetic resonance imaging revealed abnormal blood flow at the left cavernous sinus. However,because of spontaneous remission of her symptoms, she received a follow-up examination.
In 2014, she presented with prominent tortuous corkscrew vessels and exophthalmos of her left eye (Figure 1A). Her visual acuity was 20/25 OD and 20/20 OS, and the intraocular pressure was 12 mm Hg OD and 23 mm Hg OS. Hertel exophthalmometry revealed proptosis of 13 mm OD and 18 mm OS. The pupils appeared grossly normal. Slit-lamp biomicroscopic examination revealed a shallow anterior chamber in the left eye. However, no inflammatory cells were observed in the anterior chamber or vitreous cavity in either eye. Fundus examination revealed venous dilatation and tortuosity in the left eye (Figure 1B, yellow arrows). Spectral domain optical coherence tomography (SD-OCT) revealed CFs in the left eye (Figure 1C, yellow arrows).
Magnetic resonance imaging revealed swelling of the left medial, inferior, and superior rectus muscles (Figure 2A,yellow arrows) and enlargement of the superior ophthalmic vein (Figure 2B, yellow arrow). No posterior scleral thickening was detected in both eyes. Cerebral angiography revealed the presence of left-sided CCF (Figure 3A, 3B, red arrows), which was supplied by the right ECA (Figure 3A, yellow arrow) and the right ICA (Figure 3B, yellow arrow). After we performed endovascular coil embolization on the detected CCF 3wk after angiography (Figure 3C, red arrow), flow from the ECA(Figure 3D, yellow arrow) and ICA (Figure 3E, yellow arrow)was no longer detectable (Figure 3D, 3E, red arrows).
Moreover, after embolization, her left ocular corkscrew vessels were noticeably improved (Figure 4A). After embolization, her visual acuity was 20/20 OD and 20/25 OS, and the intraocular pressure was 12 mm Hg OD and 12 mm Hg OS. Hertel exophthalmometry showed improved proptosis of 12 mm OD and 14 mm OS. Slit-lamp biomicroscopic examination revealed a deep anterior chamber in her left eye. At 6mo after the procedure, dilation and tortuosity of the retinal veins had improved noticeably (Figure 4B), and SD-OCT revealed resolution of the CFs (Figure 4C).
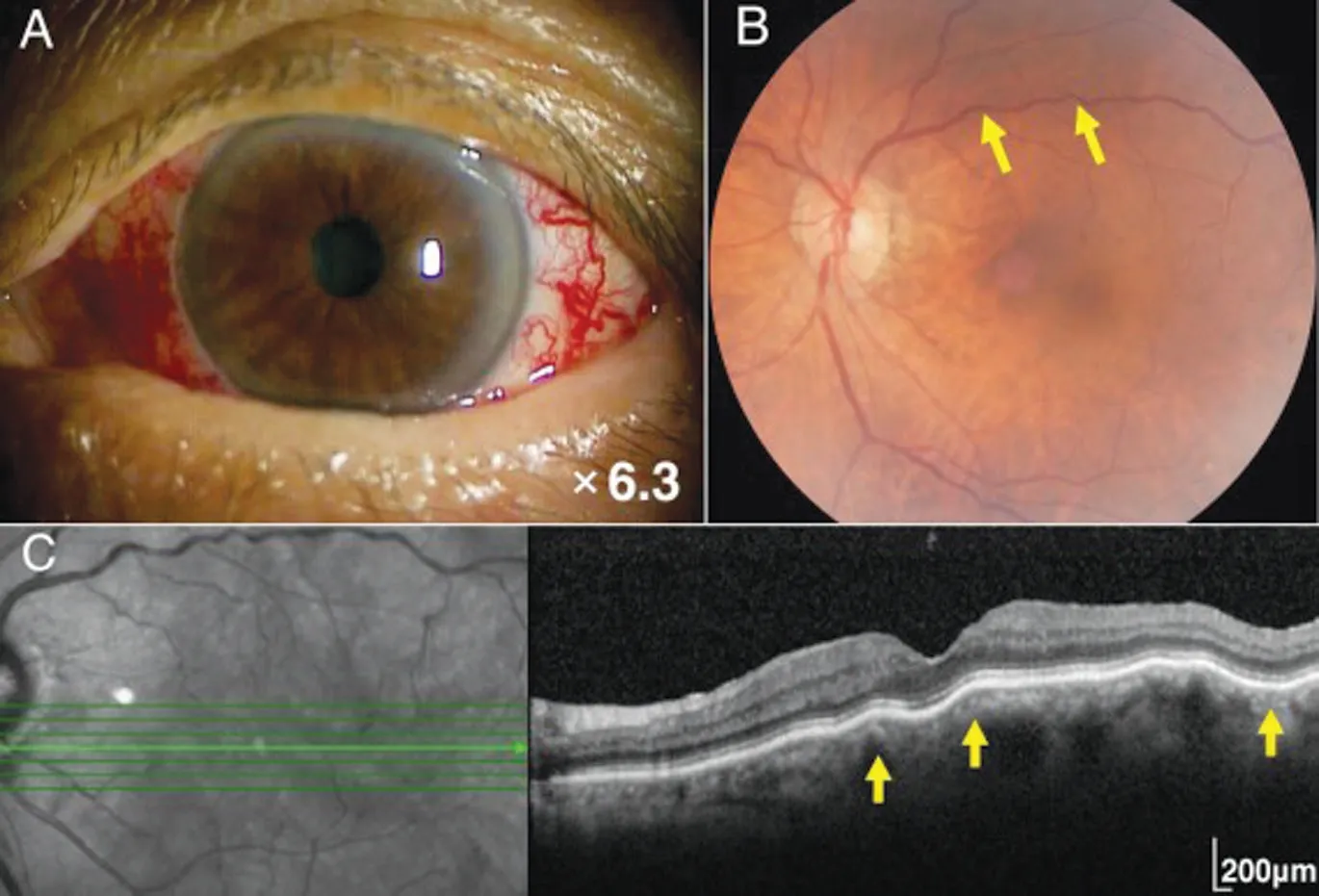
Figure 1 Preoperative ocular findings of the left-sided carotid cavernous fistula Tortuous corkscrew vessels on the left ocular surface (A). Fundus photograph showing retinal venous dilatation and vessel tortuosity in the left eye (B, yellow arrows). Spectral domain optical coherence tomography showing the choroidal folds in the left eye (C, yellow arrows).
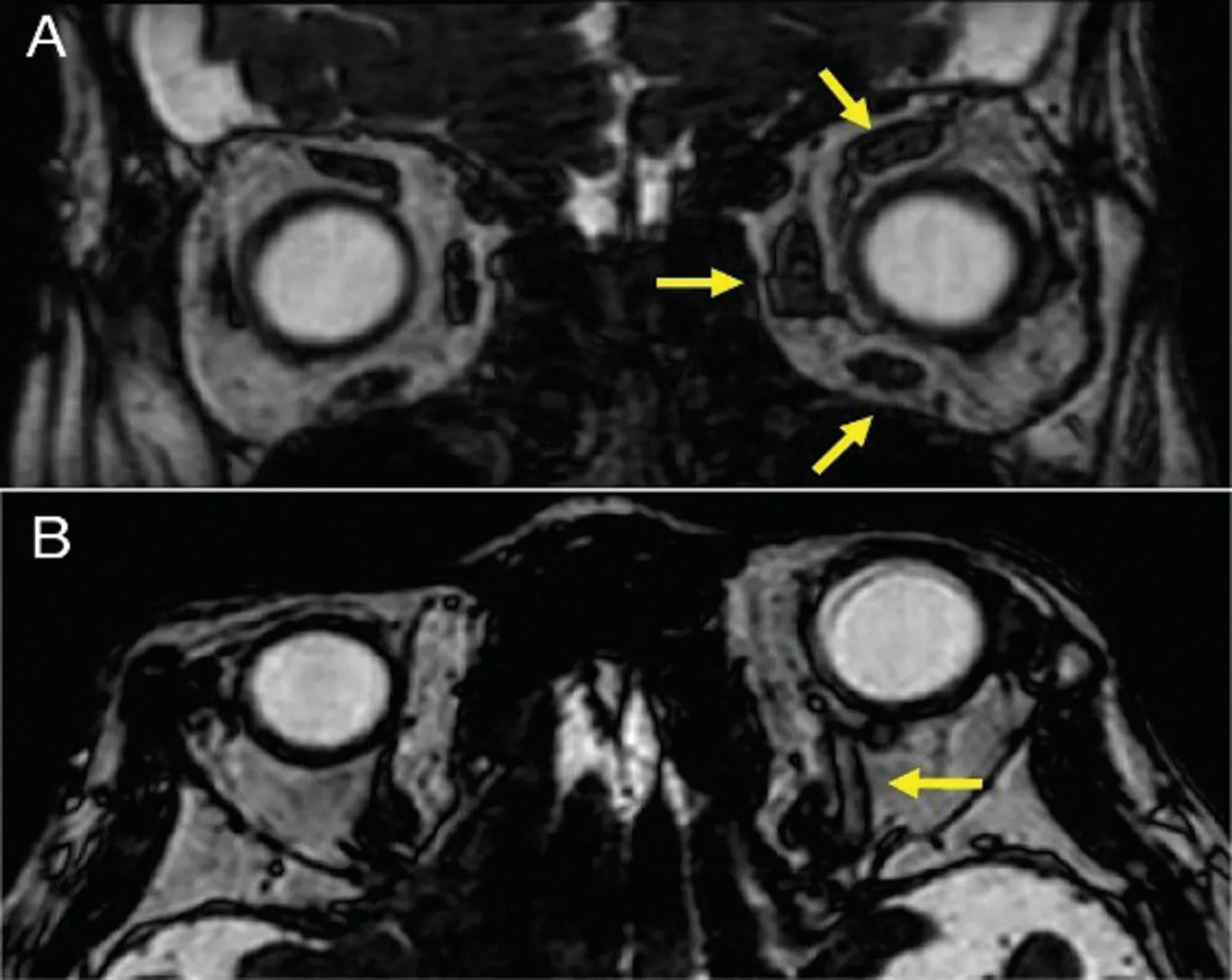
Figure 2 T2 enhanced magnetic resonance images Axial view showing swelling of the left medial, inferior, and superior rectus muscles (A, yellow arrows). Coronal view showing enlargement of the left superior ophthalmic vein (B, yellow arrow).
DISCUSSION
CCF is a term that encompasses all forms of communication,both direct and indirect, between the cavernous sinus and the carotid blood flow. This shunting may ultimately lead to numerous severe ocular manifestations that warrant close monitoring until its spontaneous closure or a prompt response if it remains patent. To our knowledge, this report is the first to detail the clinical course of concomitant CFs and CCF managed by embolization of the detected fistula. CCF presents with clinical signs, such as ocular corkscrew vessels, that can be detected during routine ophthalmic examinations, but it is often misdiagnosed as conjunctivitis or the symptoms are neglected as non-specific[19-20]. This results in delayed care and suboptimal prognosis, leading to severe complications,such as ophthalmoplegia, acute secondary glaucoma, or central retinal vein occlusion[20-21]. Hence, improving the understanding of CCF as a possible cause of CFs is important for ophthalmologic health providers in order to recognize and prevent complications of CCF.
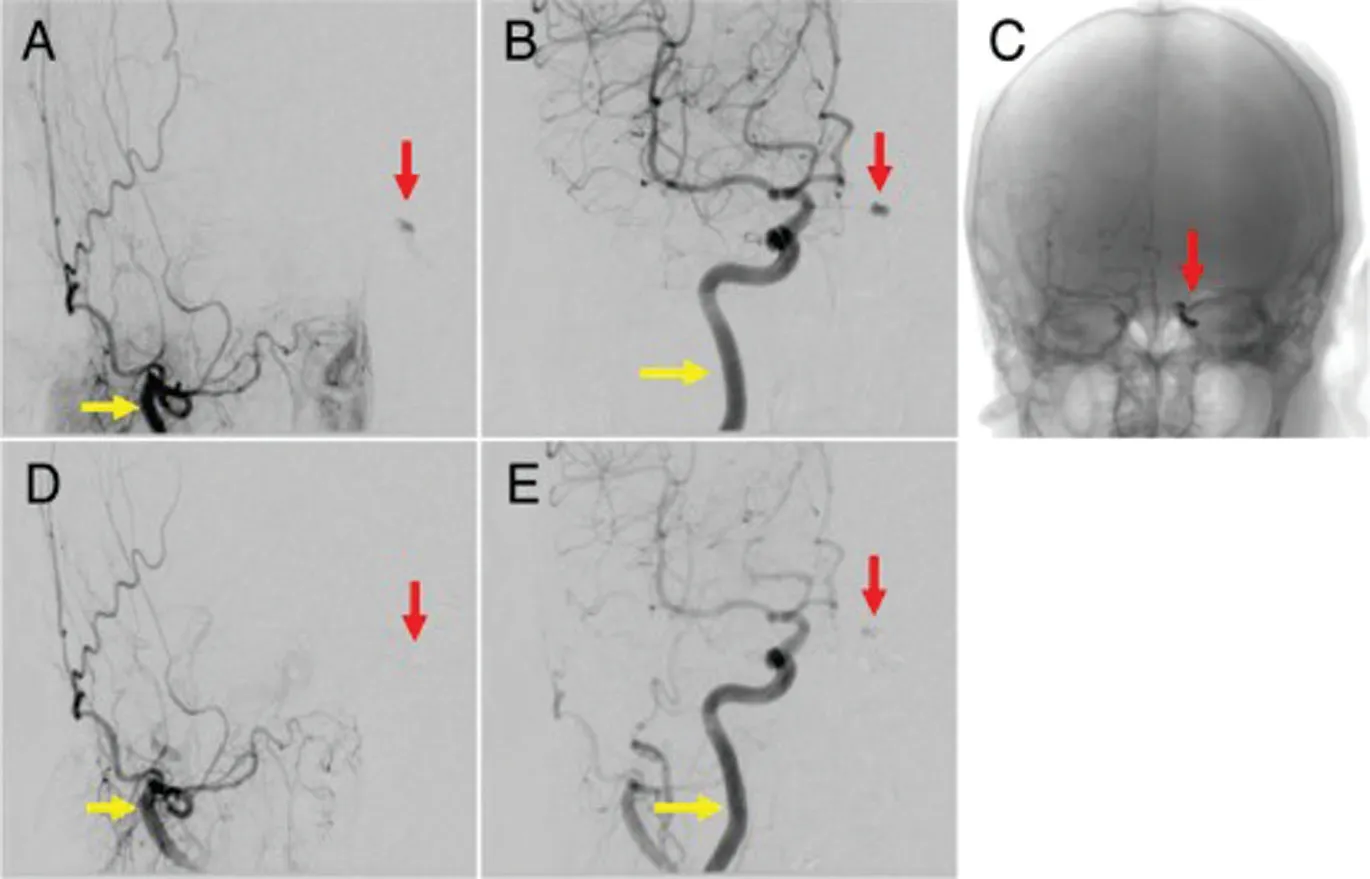
Figure 3 Frontal views of transvenous embolization The left-sided carotid cavernous fistula (CCF) (A, B, red arrows) is supplied by the right external carotid artery (A, yellow arrow) and the right internal carotid artery (B, yellow arrow). After coil embolization of the left CCF (C, red arrow), blood flow from the right external carotid artery(D, yellow arrow) and the right internal carotid artery (E, yellow arrow) is undetectable (D, E, red arrows).
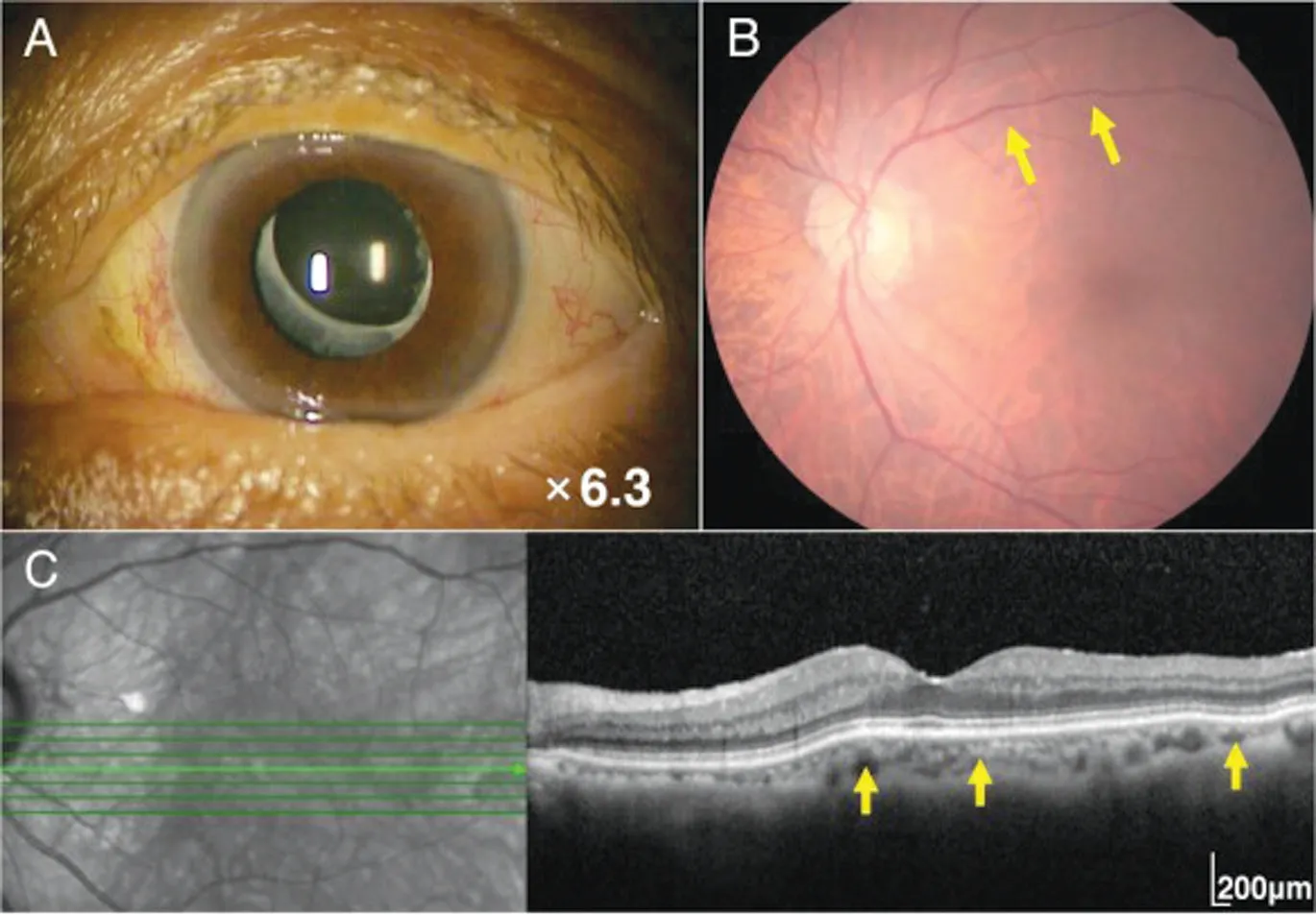
Figure 4 Postoperative ocular findings of the left-sided carotid cavernous fistula One month after embolization of the carotid cavernous fistula, tortuous corkscrew blood vessels on the left ocular surface are significantly reduced (A). Six months after embolization,retinal venous dilatation and vessel tortuosity are improved (B, yellow arrows). Six months after embolization, the choroidal folds are also improved (C, yellow arrows).
In this report, we describe in detail the presentation and clinical course of concomitant CFs and Barrow type D CCF.The mechanism by which CCF may induce CFs, as well as pachychoroid disease, likely involves venous stasis and the resultant increased vortex (vorticose) vein pressure brought on by the fistula. Based on this logic, the increased shunted blood flow from high-flow CCFs (type A) may cause more severe venous stasis, and hence, may increase the likelihood of pachychoroid disease and CFs. The current case findings also provide clinical support for the correlation between fistular blood flow and CFs, as underscored by the improvement of CFs after embolization. Notably, however, currently available reports of pachychoroid disease and CFs induced by CCF,including ours, describe cases of low-flow CCFs (types B or D) confirmed using cerebral angiography[5-7]. This suggests that a long term of choroidal circulation disorder according to slow increased vortex vein pressure caused by low-flow CFFs induces CFs. Therefore, additional research and reports investigating the relationship between CCF blood flow and choroidal changes are warranted to confirm their correlation and mechanism.
Our case report describes a patient with a history of Beh?et disease. To date, only one report has described the simultaneous presence of Beh?et disease and CFs, in which the CFs were a complication of Beh?et-related posterior scleritis[22]. Nevertheless, no clinical signs of posterior scleritis were noted in our patient, and this minimizes the likelihood that the observed CFs were scleritis-induced. However, Beh?et disease has been found to increase the likelihood of choroidal thickening in affected patients than in controls[23-24]. Currently,this choroidal thickening is considered to result from increased choroidal blood flow, increased vessel resistance, and choroidal effusion resulting from inflammation[23]. Because choroidal thickening ultimately reflects hemodynamic stasis,the simultaneous presence of CCF and Beh?et disease may synergistically predispose the affected individual to CFs.
This report has several limitations. As this report represents a single example of an uncommon disease, the results should be generalized with caution. CCF is a rare phenomenon[2], and large-scale studies on its pathology and treatment options are currently lacking. Besides, we did not utilize enhanced depth imaging optical coherence tomography (EDI-OCT) to measure choroidal thickness. While SD-OCT is a viable tool for confirming gross choroidal thickening and monitoring changes before and after treatment, its measurement accuracy remains questionable. A previous report described choroidal thickness improvement after spontaneous closure of the fistula[6]; hence,future case reports on CCFs should utilize EDI-OCT to accurately measure and track choroidal thickness changes.
In conclusion, we report a rare case of CF improvement following CCF embolization. Delayed CCF intervention may lead to irreversible ophthalmoplegia, secondary glaucoma,and/or central retinal vein occlusion. Understanding CCF as a risk factor for CFs is crucial to avoiding these complications and improving patient prognosis.
ACKNOWLEDGEMENTS
The authors thank the orthoptists at Juntendo University Hospital, Department of Ophthalmology, for collecting and measuring the data for the case report.
Conflicts of Interest: Akasaki Y, Johnson & Johnson Vision
Care;Inomata T, Johnson & Johnson Vision Care, Inc.; Hogy Medical Co., Ltd.; SEED Co., Ltd.; Santen Pharmaceutical Co., Ltd.; Alcon Japan Ltd.; and Lion Ltd.; Santen Pharmaceutical Co., Ltd.; InnoJin Co., Ltd.;Sung J,None;Ito M,None;Kobayashi H,None;Kuwana R,None;Oishi H,None;Murakami A,Eisai Co., Ltd.; Kowa Ltd.; Novartis Pharma K.K.; HOYA Corporation; ROHTO Pharmaceutical Co., Ltd.; Alcon Japan Ltd.; Alcon Pharmaceuticals Ltd.;AMO Japan K.K.; Otsuka Pharmaceutical Co., Ltd.; SEED Co., Ltd.; Senju Pharmaceutical Co., Ltd.; Residence Building Management, Ltd.; Santen Pharmaceutical Co., Ltd.; Johnson& Johnson Vison Care and Alcon Japan Ltd.
 International Journal of Ophthalmology2022年11期
International Journal of Ophthalmology2022年11期
- International Journal of Ophthalmology的其它文章
- Paracentral acute middle maculopathy after diagnostic cerebral angiography in a patient with a contralateral carotid-cavernous fistula
- Optical coherent tomography angiography observation of macular subinternal limiting membrane hemorrhage in CO poisoning: a case report
- Role of the ultra-wide-field imaging system in the diagnosis of pigmented paravenous chorioretinal atrophy
- A rare combination of late-onset post-LASlK keratectasia after early-onset central toxic keratopathy
- Gastrointestinal microbiome and primary Sj?gren’s syndrome: a review of the literature and conclusions
- lnsights on the possibility of SARS-CoV-2 transmission through the eyes
