Gastrointestinal microbiome and primary Sj?gren’s syndrome: a review of the literature and conclusions
Yu-Feng Yao, Mei-Ying Wang, Xiao-Yan Dou
1Department of Ophthalmology, Shenzhen Second People’s Hospital, the First Affiliated Hospital of Shenzhen University,Shenzhen 518035, Guangdong Province, China
2Shantou University Medical College, Shantou 515031, Guangdong Province, China
3Department of Rheumatology and Immunology, Shenzhen Second People’s Hospital, the First Affiliated Hospital of Shenzhen University, Shenzhen 518035, Guangdong Province,China
Abstract
● KEYWORDS: gastrointestinal microbiome; primary Sj?gren’s syndrome; dry eye; dysbiosis; autoimmunity;clinical therapy
INTRODUCTION
The human gastrointestinal microbiome (GM), which consists of bacteria, fungi, viruses, and protozoa[1],contains approximately 4 trillion microorganisms[2]and 150 000 microbial genomes[3], making it the largest known commensal microbial community in the human body. It has been symbiotic with humans for millions of years and plays an important role in regulating the human immune system[2].Colonization by the GM helps to stimulate the intestinal epithelia to secrete mucus and firm the intercellular tight junctions to ensure the completeness of the gut mucosal barrier[4-5]. In addition, the pattern-recognition receptors expressed by the intestinal epithelia identify the microbiotaassociated molecular patterns of commensal bacteria to adjust their interactions[4-5]. The GM can enhance the killing of pathogens by neutrophils and regulate the proliferation and differentiation of lymphocytes through their metabolites[5-7].For example, segmented filamentous bacteria induce Th17 cells to secrete the pro-inflammatory cytokine interleukin-17(IL-17) through serum amyloid A (SAA) produced by epithelia or signal transducer and activator of transcription 3 (STAT3) in epithelia[8], while polysaccharide A fromBacteroides fragilisactivates Toll-like receptor 2 (TLR2) on forkhead box P3(FOXP3)-positive Treg cells to induce them to secrete antiinflammatory cytokine IL-10 to antagonize the immune effect of IL-17 or other pro-inflammatory cytokines[9], which helps maintaining the balance of the immune system[5]. GM dysbiosis may break the balance and lead to primary Sj?gren’s syndrome(pSS), which may be connected with chronic local gut mucosal inflammation, molecular mimicry, and other mechanisms[10].pSS is a systemic autoimmune disease most common in middle-aged women[11]. It affects exocrine glands and often causes dryness of the eyes or mouth, often affecting other organs such as the kidneys and lungs[12]. Its pathogenesis may be related to the activation of the immune system due to viral stimulation, which leads to the production of autoantibodies[12].The immune complexes formed by autoantibodies maintain and amplify the immune response through interferon alpha(IFN-α) to cause tissue damage[12]. Anti-Ro/SSA or anti-La/SSB autoantibodies are often detected in the serum of patients[12].Lymphocyte or autoantibody infiltration is often observed in exocrine gland biopsies[12]. Dry eye associated with pSS has a serious impact on the quality of life and work efficiency of patients[13], and the economic burden caused by medical expenses cannot be ignored[14]. At present, the management of dry eye associated with pSS is mainly symptomatic, and immunotherapy is still under development[12,14]. However,considering the high economic cost[15]and serious side effects[16]of immunotherapy, the development of a low-cost,safe, and effective therapy is urgently needed.
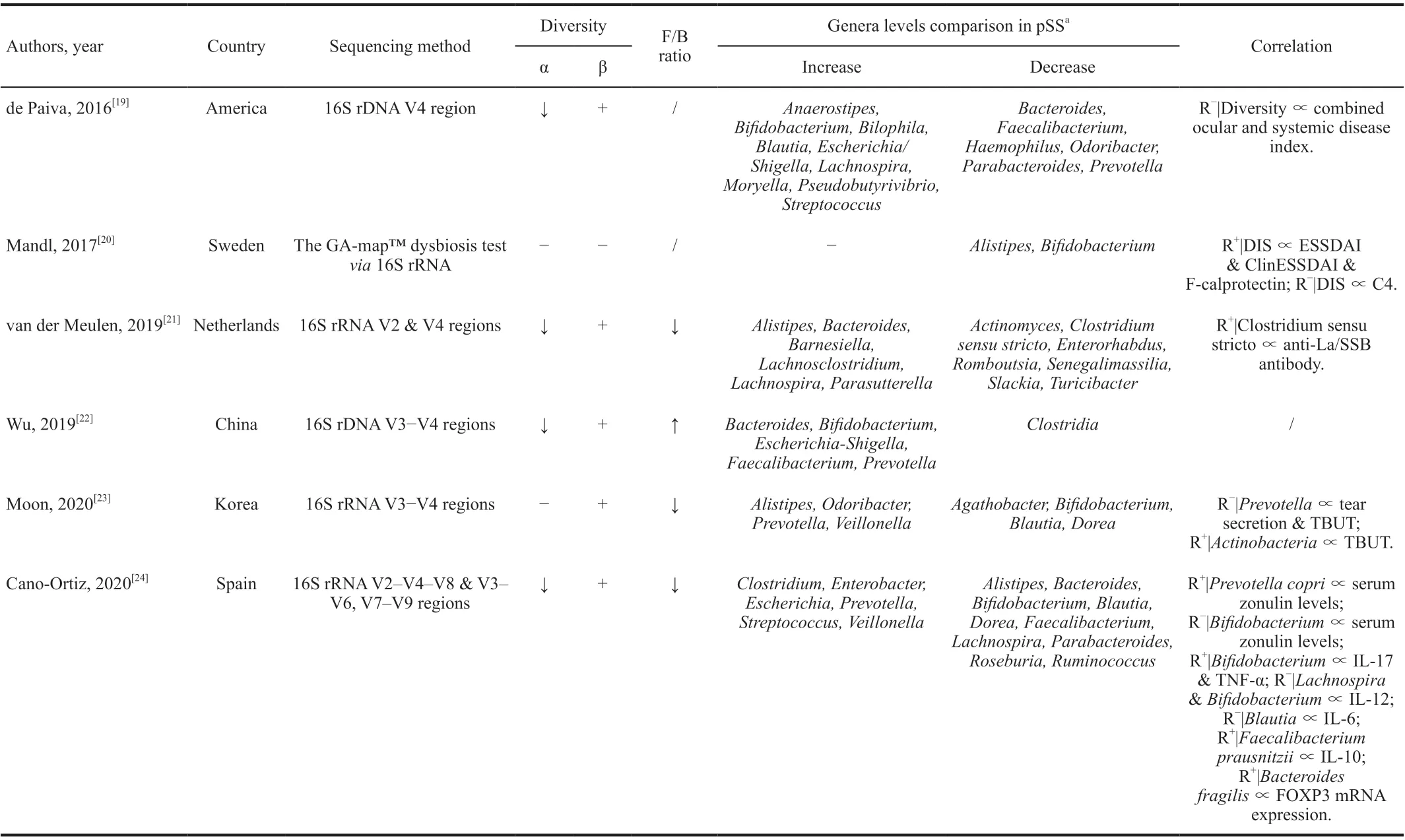
Table 1 Human studies about the gastrointestinal microbiome in primary Sj?gren’s syndrome
In recent years, the human GM has been a hot topic in the field of immune research, so we hope to draw some inspiration from such research to guide the development of new therapies targeting the GM for dry eye associated with pSS. In this review, we first briefly describe the characteristics of GM in patients with pSS compared with healthy individuals. Then,we summarize the possible mechanisms that explain how GM dysbiosis contributes to pSS, based on existing research.Finally, we discuss the implications for future clinical research and applications.
GASTROINTESTINAL MICROBIOME IN PATIENTS WITH PRIMARY SJ?GREN’S SYNDROME
The human GM is mostly composed of theFirmicutesandBacteroidetesbacterial phyla, and is affected by various factors such as race, age, region, diet,etc[17]. The relative abundance of species (or even phyla) differs greatly from person to person, so it is difficult to define the standard composition of a healthy microbiota[18]. Moreover, due to the complex functions of microorganisms and their interactions, it is difficult to establish whether a specific microbe is beneficial or harmful[18].Presently, there are few studies comparing the characteristics of the GM between patients with pSS and healthy individuals,and their results vary greatly[19-24](Table 1).
According to previous studies, the α-diversity of the GM is decreased[19,21-22,24], and its β-diversity significantly altered[19,21-24], in patients with pSS compared with healthy individuals. A correlation was observed between GM diversity and dry eye symptom severity[1-20,23]. As a possible indicator of GM dysbiosis[25-26], theFirmicutes/Bacteroidetes(F/B) ratio is often observed to be decreased in patients with autoimmune diseases[27-28]. At least three studies observed a decreased F/B ratio in patients with pSS[21,23-24]. For specific genera,the results of different studies vary significantly, which may be explained by differences in race, gender, or diet among the cohorts, small sample sizes, and different sequencing methods. Therefore, it is difficult to identify the target bacteria of pSS.Bifidobacterium,Faecalibacterium,Blautia, andBacteroidesare butyrate-producing genera in the human body[29-31]. Butyrate provides energy for colonic epithelia to maintain the gut mucosal barrier and inhibits the activation of nuclear factor kappa-B (NF-κB) in immune cells by binding to G-protein coupled receptors to inhibit the secretion of tumor necrosis factor alpha (TNF-α), IL-6, and IL-12[29,32-33].Moreover, butyrate could downregulate the expression of the pathological cytokine IL-17 and promote Treg proliferation to inhibit inflammation[34-35].Faecalibacteriumwas found to be positively correlated with IL-10 serum levels in patients with pSS[24], and the link betweenFaecalibacteriumand inflammation has been well demonstrated in other studies[31]. The abundance ofFaecalibacteriumdeclined in Crohn’s disease and other intestinal diseases associated with inflammation[36-37], and recovered after anti-inflammatory treatment[38-39]. Therefore,Faecalibacteriummay be an indicator of human gut health. Another genus,Bifidobacterium,was found to be negatively correlated with elevated serum levels of zonulin[24], a protein that acts on the tight junctions between intestinal epithelia and breaks them down[40]. Elevated serum levels indicate increased intestinal permeability[40]. A decreased abundance ofBifidobacteriahas also been observed in other rheumatic immune diseases[41-42], suggesting the possibility of developing a probiotic therapy. It has been shown that autoimmune dry eye mice treated with probiotics have improved corneal defects, increased tear secretion, decreased T cell infiltration, and increased Treg circulating flux[43-44]. As a key genus in chronic inflammatory diseases[45-46],Prevotellacontains key enzymes for mucin degradation, which destroys the colonic mucosal barrier and increases gut permeability[47].Prevotellaalso reduces the level of short-chain fatty acids(SCFAs) and exacerbates intestinal mucosal inflammation[48].Prevotellawas found to be positively associated with elevated serum zonulin levels[24]and negatively associated with tear secretion and tear break-up time (TBUT) in patients with pSS[23], suggesting the presence of a “GM-lacrimal glandocular surface” axis. Correspondingly, most studies found thatBifidobacterium[20,23-24],Faecalibacterium[19,24],Blautia[23-24], andBacteroides[19,24]decreased, whilePrevotellaincreased[22-24], in patients with pSS. These findings suggest that GM dysbiosis in patients with pSS may lead to a decrease in intestinal barrier function, which causes increased intestinal permeability,leading to local intestinal mucosal inflammation and creating a chronic pro-inflammatory environment in the body. These risk factors may induce pSS under certain conditions, leading in turn to lacrimal gland injury[49].
However,Lachnospirais also a butyrate-producing genus[29-30]which, in contrast, was observed to increase in patients with pSS in at least two studies[19,21], andBifidobacteriumwas positively correlated with serum levels of IL-17 and TNF-α[24], suggesting that GM changes in patients with pSS are complicated and irregular. This could be explained by the variability of GM, different sequencing methods, and high false positive rates caused by the small sample sizes of the above studies. In addition, these studies were all cross-sectional and could not reflect the dynamic process of GM changes in the development of pSS. Therefore, future research should increase the sample size and compare samples longitudinally.Moreover, microbiota from other body parts, such as the oral cavity or the ocular surface, and other components of the GM,such as fungi, viruses, and protozoa, should be investigated to completely reflect the composition characteristics of the human microbiota in pSS. It should be pointed out that although studies on the composition characteristics of the GM in patients with pSS provide preliminary support for the impact of GM on pSS, there is no clear indication of the causality between GM and disease development, since GM dysbiosis may simply reflect the adaptation of the microbiota to the pathological state. Therefore, mechanistic studies should be conducted to further elucidate the key interaction pathways in the “GMlacrimal gland-ocular surface” axis, and provide inspiration for the clinical therapy of dry eye associated with pSS.
POSSIBLE MECHANISMS OF GASTROINTESTINAL MICROBIOME CONTRIBUTION TO PRIMARY SJ?GREN’S SYNDROME
Many animal studies have revealed a connection between GM and pSS. Compared with normal mice, germ-free mice tended to develop dry eye and were accompanied by pSS-like symptoms such as reduced density of conjunctival goblet cells,lacrimal lymphocyte infiltration, and increased circulation levels of autoreactive T lymphocytes[49-50]. Here, we summarize the possible mechanisms that explain how GM dysbiosis contributes to pSS, based on existing research (Figure 1).
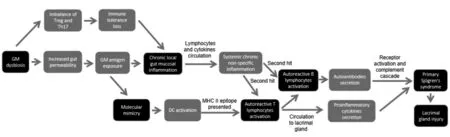
Figure 1 How gastrointestinal microbiome contributes to primary Sj?gren’s syndrome.
Molecular MimicryLong-term GM dysbiosis may result in increased intestinal permeability[24], which may increase the risk of GM antigen exposure and autoantibody production[51].The exposed GM antigens are recognized and presented as MHC class II antigens by dendritic cells, which ultimately activate specific CD4+ T lymphocytes[49]. The main mechanism involved is “molecular mimicry”[52]. Human Ro60 reactive T lymphocytes can react with Ro antigens and cross-react with von Willebrand Factor A (vWFA) and peptides containing the Trove domain[53]. These mimicking peptides are found in the human GM such asBacterioides finegoldii,Bacterioides intestinalis,Bacterioides fragilis, andAlistes finegoldii[53].When these microorganisms or their antigens are exposed,Ro60 reactive T lymphocytes may be activated through“molecular mimicry”[53]. The activated specific autoreactive T lymphocytes reach the exocrine glands, such as the lacrimal gland, through the blood circulation, and secrete IFN-γ, IL-12,IL-17, and other inflammatory cytokines, leading to lacrimal gland injury and inducing dry eye[49,51]. On the other hand,activated specific autoreactive T lymphocytes can promote the proliferation of plasma cells, which secrete autoantibodies such as Ro/SSA or La/SSB[12]. These autoantibodies recruit inflammatory cells such as macrophages to infiltrate the lacrimal glands and induce dry eye through receptor activation and the complement cascade[12]. Several studies have immunized mice with Ro polypeptides, and pSS-like symptoms were observed in these mice[53-55]. In addition, CD4+ T lymphocytes,various pro-inflammatory cytokines and autoantibodies, and abnormally high circulating levels of CD4+ T lymphocytes and B lymphocytes were found in the lacrimal glands of several mouse models of autoimmune dry eye[19,49-50,56]. Interestingly,Coxsackie virus 2B peptides also cross-react with anti-Ro/SSA antibodies[57], and IFN-α serum levels in patients with pSS were often elevated[12], suggesting that viral infection plays an important role in the development of pSS.
Chronic Local Gut Mucosal InflammationExposure to GM antigens caused by long-term GM dysbiosis causes the local intestinal mucosa to promote inflammation[51]. As proinflammatory lymphocytes and cytokines are released into circulation, they may lead to systemic chronic nonspecific inflammation, giving the body the potential to activate autoreactive T or B lymphocytes to induce autoimmunity[51,58].Researchers have found that mice would develop chronic nonspecific inflammation of the exocrine glands when exposed to chronic stimulation withEscherichia coliouter membrane protein A (OmpA) or flagellin (FliC), a flagellar filament structural protein[56,59]. Additionally, pSS-like symptoms were observed[56,59]. Moreover, since many kinds of microorganisms in the GM and their metabolites could maintain the state of immune tolerance by balancing the circulating fluxes of Treg and Th17, GM dysbiosis would result in the loss of intestinal immune tolerance because of the imbalance of circulating levels of Treg and Th17 (usually the decrease of FOXP3+Treg or the increase of Th17)[5], and induce chronic local gut mucosal inflammation, which ultimately causes systemic chronic non-specific inflammation[10,49,51]. Correspondingly,GM dysbiosis and the imbalance between Treg/IL-10 and Th17/IL-17 were observed in the cornea, lymph nodes, and serum of several mouse models of autoimmune dry eye[19,43-44].Increased serum levels of IL-17, IL-6, IL-12, and TNF-α,decreased IL-10 serum levels, and reduced expression of FOXP3 mRNA in peripheral blood monocytes were also observed in patients with pSS[24]. However, systemic chronic non-specific inflammation may require a “second hit,” such as stress, viral infection,etc., to trigger the critical activation of autoreactive T or B lymphocytes, followed by anti-Ro/SSA or anti-La/SSB antibody secretion and lacrimal gland injury[12,51,60].As mentioned above, Coxsackie virus infection may play an important role in the pathogenesis of pSS[57]. When patients with chronic GM dysbiosis are infected with the Coxsackie virus, systemic chronic non-specific inflammation in the body may enable the virus to amplify and maintain the autoimmune response through IFN-α and cause the occurrence of pSS[12,51].These elements of evidence suggest the existence of a “GMlacrimal gland-ocular surface” axis, but the signal pathways or possible molecular mechanisms involved in each link of the axis still need to be confirmed and elucidated.
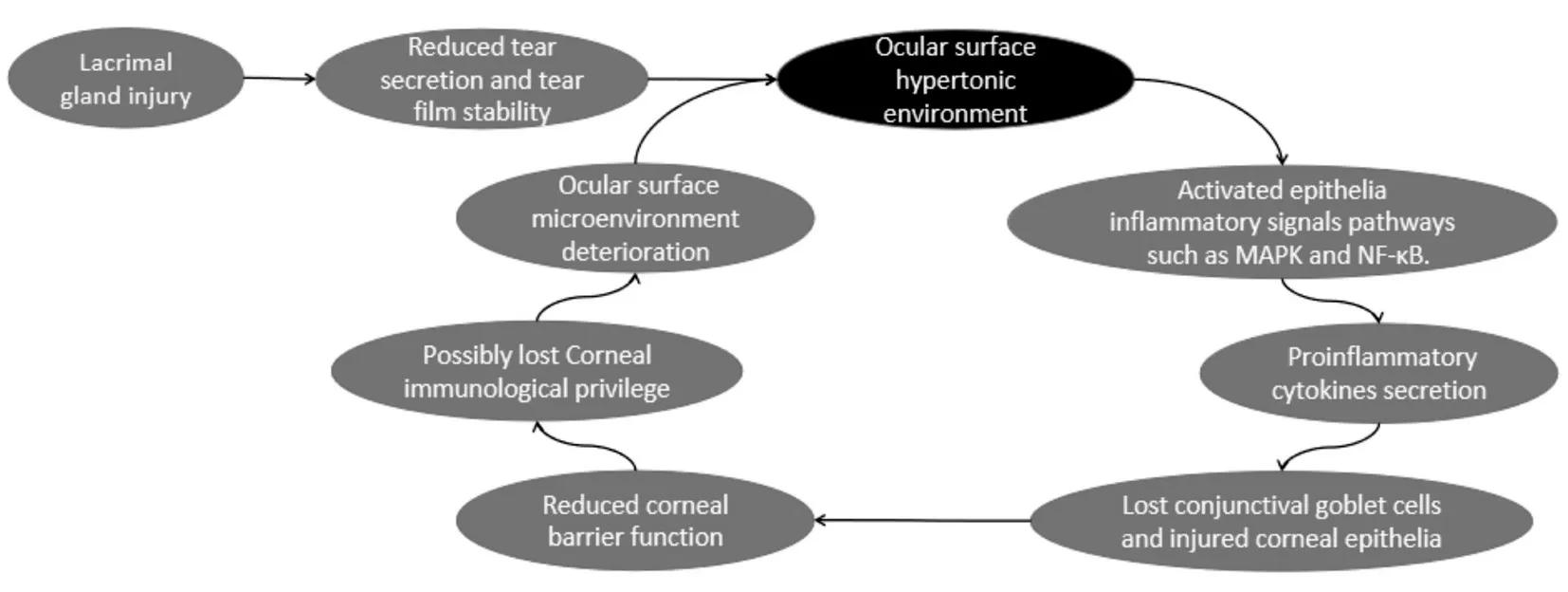
Figure 2 The key role of ocular surface hypertonic environment in dry eye disease.
When GM dysbiosis causes lacrimal gland injury through the aforementioned mechanisms, the reduction of tear secretion and stability of the tear film results in a hypertonic ocular surface environment in patients with pSS[60]. An ocular surface hypertonic environment, which is considered to be the central mechanism inducing dry eye[60], can activate inflammatory signals such as mitogen-activated protein kinase (MAPK)and NF-κB pathways in the ocular surface epithelia, and then release inflammatory cytokines such as IL-1α, IL-1β,and TNF-α, causing the loss of conjunctival goblet cells and injury of corneal epithelia[61-62]. The loss of corneal barrier function caused by corneal epithelial cell injury makes it easy for blood or lymphatic vessels from the conjunctiva to invade the corneal surface, ultimately resulting in the loss of corneal immunological privilege[63]. Moreover, it further aggravates autoimmune inflammation and the microenvironment of the ocular surface, finally causing symptoms such as dry eye,foreign body sensation, and visual fatigue in patients with pSS[60,62,64](Figure 2).
FUTURE CLINICAL THERAPIES
If the relationship between GM and pSS could be further elaborated and proven, or target microorganisms in the pathogenesis of pSS could be identified, the clinical therapy for pSS would be subverted and simplified, just as the discovery ofHelicobacter pyloriled to a landmark change of clinical therapy for peptic ulcer[65]. However, it is extremely difficult to identify the target microorganisms owing to the susceptibility of the GM to dynamic changes and its variability among individuals[18]. However, we could still obtain some inspiration from the therapy for GM dysbiosis described in previous studies[22,43-44,49-50,66-67](Table 2).
Diet is the most economical intervention, and it significantly affects the GM[17]. It may be the best preventive measure for GM-related diseases in the future. Kawashimaet al[66]observed that dietary supplements containing SCFAs, lactoferrin,vitamins, and other nutrients improved the symptoms of rats and patients with dry eye. Hydrogen-producing milk could improve the stability of the tear film, but not tear secretion,in patients with dry eye, and there were gender differences in its efficacy[67]. It should be noted that the formulation of a dietary therapy for GM dysbiosis is complicated, because it is extremely difficult to determine the specific types or amounts of nutrients needed to obtain satisfactory efficacy.
Antibiotic or probiotic-based therapies are easy to implement.However, if the target microorganisms have not been precisely identified, antibiotics may themselves cause GM dysbiosis and aggravate the symptoms[50]. The efficacy of probiotics is limited by their susceptibility to gastric acids, which causes low success rates of intestinal colonization[68]. Currently, it is not suitable as the main therapy for patients with pSS, although its efficacy has been proven in animal experiments[43-44].
Another option is fecal microbiota transplantation. It has achieved remarkable efficacy in ulcerative colitis[69]and refractory infections caused byClostridium difficile[70].Improved symptoms were observed in mouse models of autoimmune dry eye after fecal microbiota transplantation[50].However, its safety remains uncertain owing to the lack of long-term follow-up data[71]. Moreover, patients may easily get infected by some pathogens, such asCytomegalovirus, during transplantation[72]. In addition, the negative psychological effects caused by fecal microbiota transplantation should not be ignored.
It is worth mentioning in this context traditional Chinese medicine.Studies have revealed that traditional Chinese medicine is closely connected to the GM[73-74]. Some specific Chinese medicines could increase the content of SCFAs to promote the proliferation of probiotics and adjust GM dysbiosis[73-74].Traditional Chinese medicine also repairs the morphology and function of gut epithelia, improves gut absorption capacity, and strengthens the gut mucosal barrier[74]. Studies have reported that the symptoms and GM dysbiosis in patients with pSS were significantly improved after taking Yangyin Yiqi Huoxue recipe[22]. However, the reason why traditional Chinese medicine therapy is currently difficult to be recognized is that its theoretical basis, which refers to “qi and blood in meridians” is hard to prove[75]. A large number of clinical trials are necessary to confirm their safety and efficacy[75].

Table 2 Efficacy of interventions on dry eye in animal and human studies
CONCLUSION
As the largest commensal microbial community in the human body, GM affects human health and disease in terms of immunity and metabolism. We believe that further research on the human GM will show it to be a key factor in determining human health and disease. The correlation between GM dysbiosis and dry eye associated with pSS is generally observed in human studies, but it is not clear whether GM dysbiosis is the cause or the result of the disease,due to the lack of mechanistic studies in humans. Animal studies have provided evidence that GM plays a causal role in the pathogenesis of pSS, but caution should be used when applying their conclusions to humans due to the different genera involved in GM dysbiosis in humans, different methods used to construct animal models of dry eye, and the inability of model construction to represent the pathogenesis of pSS.Some animal studies have demonstrated the effectiveness of therapies such as probiotics and fecal bacteria transplantation for dry eye associated with autoimmunity. However, largescale human clinical studies and mechanistic studies are still needed to provide strong data on the safety and efficacy of new therapies aimed at the GM.
ACKNOWLEDGEMENTS
Foundations:Supported by the Science and Technology Program for Basic Research in Shenzhen (No.JCYJ20200109140412476);Clinical Research Project of Shenzhen Second People’s Hospital in 2019 (No.20193357009).
Conflicts of Interest: Yao YF,None;Wang MY,None;Dou XY,None.
 International Journal of Ophthalmology2022年11期
International Journal of Ophthalmology2022年11期
- International Journal of Ophthalmology的其它文章
- Paracentral acute middle maculopathy after diagnostic cerebral angiography in a patient with a contralateral carotid-cavernous fistula
- Optical coherent tomography angiography observation of macular subinternal limiting membrane hemorrhage in CO poisoning: a case report
- Choroidal folds associated with carotid cavernous fistula:a case report
- Role of the ultra-wide-field imaging system in the diagnosis of pigmented paravenous chorioretinal atrophy
- A rare combination of late-onset post-LASlK keratectasia after early-onset central toxic keratopathy
- lnsights on the possibility of SARS-CoV-2 transmission through the eyes
