Optical coherent tomography angiography observation of macular subinternal limiting membrane hemorrhage in CO poisoning: a case report
Heng-Di Zhang, Ling Zhang, Fei Han, Wei Jiang, Fen Lan
1Department of Ophthalmology of the General Hospital of Western Theater Command, Chengdu 610083, Sichuan Province, China
2Department of Radiotherapy of the General Hospital of Western Theater Command, Chengdu 610083, Sichuan Province, China
Dear Editor,
I am Heng-Di Zhang, from the Department of Ophthalmology of the Geberal Hospital of Western Theater Command. I am writing to present a case report on optical coherence tomography angiography (OCTA)observation of macular subinternal limiting membrane hemorrhage in carbon monoxide (CO) poisoning.
CO is the most common imperceptible but lethal gas. In the United States, CO causes more than 50 000 intoxications,while globally, the mortality rate is 4.6 per million[1-2]. Many studies have focused on CO intoxication damage to the central nervous system and cardiovascular system[3-4], but the eye remains a neglected organ in CO poisoning.
Here, we present a case using spectral-domain optical coherence tomography (SD-OCT) and OCTA to observe the vascular density and microvasculature of the macular area in CO retinopathy. The patient signed informed consent. This study was approved by the local ethics committee.
CASE PRESENTATION
A previously healthy 32-year-old male was found comatose in his bed with a burning water heater and blocked windows.When brought to the Emergency Room, his blood pressure was 119/77 mm Hg, pulse rate was 100 beats/minute,temperature was 36.5℃, and respiratory rate was 25 breaths per minute and regular. Cerebral CT scan showed no hemorrhage. Ophthalmology was not consulted until 9d after admission; at that time, the patient had aroused from his coma and complained of decreased visual acuity in his left eye. The ophthalmological examination of his right eye was unremarkable; the best corrected visual acuity (BCVA) was 20/20 in the right eye, while the visual acuity was 20/200 and uncorrectable in the left eye. Nonmydriatic fundus photography revealed several round-shaped peripapillary hemorrhages, a yellowish-red hemorrhage at the macular fovea in his left eye, and vitreous opacity. SD-OCT showed a domeshaped, highly reflective lesion under the internal limiting membrane (ILM) but could not separate it from the retinal nerve fiber layer (RNFL). Fundus angiography (FFA) and indocyanine-green angiography (ICGA) further confirmed that the hemorrhage was located on the surface of the macula, as it blocked all retinal vascular fluorescence (Figure 1).
Thus, the patient underwent 25G vitrectomy and ILM peeling in his left eye 21d after admission. The delayed operation was due to dorsal and sacral decubital ulcers, and the patient could not lay flat. The postoperative BCVA of his left eye increased to 20/32. At his 1-month follow up, SD-OCT showed increased macular thickness and a small patch of the highly reflective lesion in the outer nuclear layer (ONL), and OCTA showed decreased superficial and deep macular vascular density(SMVD 37.62%; DMVD 23.49%) in the left eye and smaller superficial and deep foveal avascular zones (superficial FAZ 0.13 mm2; deep FAZ 0.81 mm2) compared to the right eye(SMVD 42.27%; DMVD 25.53%; superficial FAZ 0.33 mm2;deep FAZ 1.03 mm2; Figure 2). At his 6-month follow up, his macular thickness had recovered, the highly reflective lesion was no longer detectable, and his visual acuity remained stable; however, the SMVD and DMVD were further reduced and deep FAZ was enlarged (SMVD 36.11%; DMVD 22.89%;superficial FAZ 0.19 mm2; deep FAZ 0.88 mm2; Figure 3).
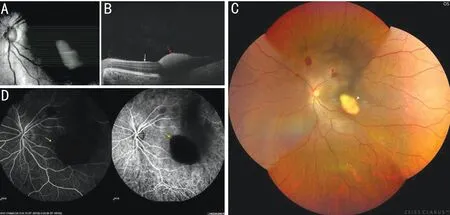
Figure 1 Preoperative fundus examinations A: Preoperative 21-line scan image of SD-OCT; B: Macula of the left eye, white arrow shows internal limiting membrane, red arrow shows elevated, highly reflective lesion that could not be distinguished from the RNFL; C: Nonmydriatic fundus photography revealed several round-shaped peripapillary hemorrhages (red arrowheads) and a yellowish-red hemorrhage right at the macular fovea (white arrowhead); D: The hemorrhage blocked all retinal vascular fluorescence in FFA and ICGA (yellow arrows). RNFL: Retinal nerve fiber layer; SD-OCT: Spectral-domain optical coherence tomography; FFA: Fundus angiography; ICGA: Indocyanine-green angiography.
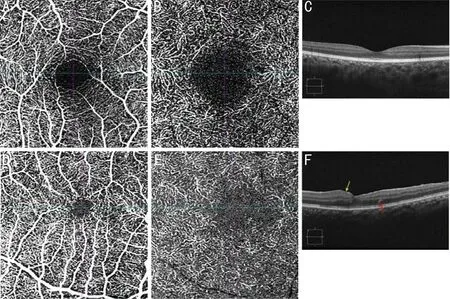
Figure 2 OCTA and SD-OCT scan at 1-month follow-up A: Superficial OCTA of the right eye; B: Deep OCTA of the right eye; C: SDOCT B-scan of the right eye; D: Superficial OCTA of the left eye showed smaller FAZ and lower flow density; E: Deep OCTA of the left eye showed irregular FAZ and decreased flow density; F: SD-OCT B-scan showed increased macular thickness (yellow arrow) and a small patch of the highly reflective lesion in the outer nuclear layer (red arrow). OCTA: Optical coherent tomography angiography; SD-OCT: Spectral domain optical coherent tomography; FAZ: Foveal avascular zone.
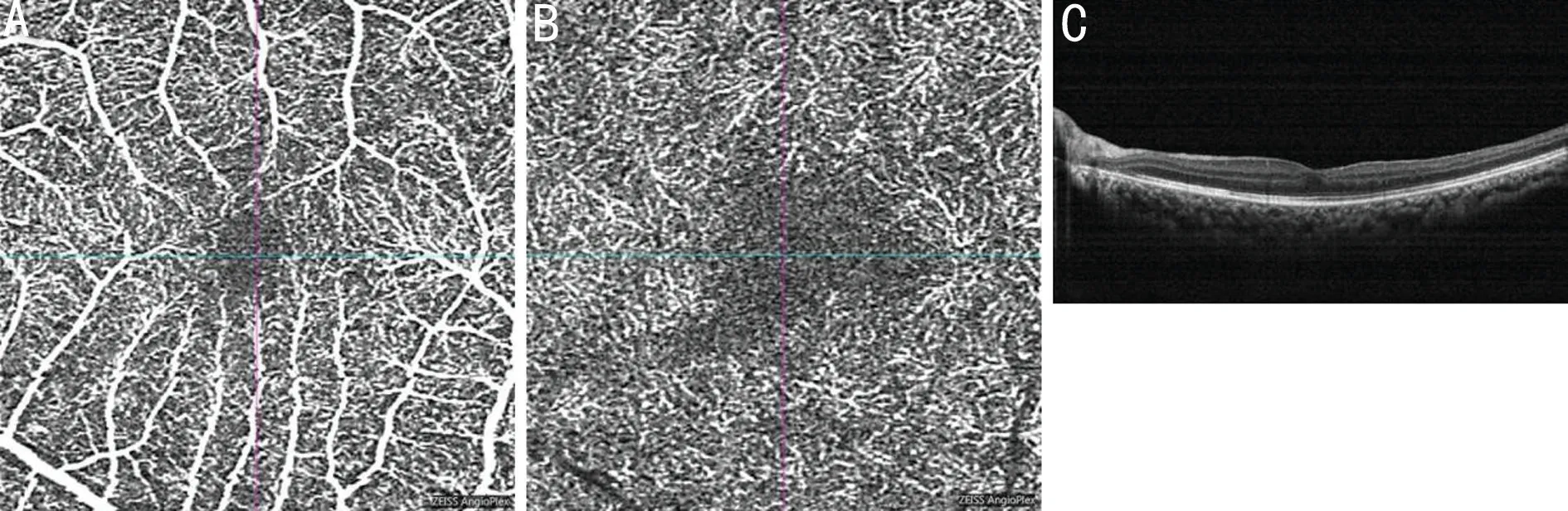
Figure 3 OCTA and SD-OCT scan at 6-month follow-up A: Superficial OCTA of the left eye showed a stable superficial FAZ and reduced flow density compared to the 1-month follow-up; B: Deep OCTA of the left eye showed an enlarged deep FAZ and decreased deep flow density compared to the 1-month follow-up; C: B-scan of the left eye showed no significant macular edema or highly reflective lesion. OCTA: Optical coherent tomography angiography; SD-OCT: Spectral domain optical coherent tomography; FAZ: Foveal avascular zone.
DISCUSSION
Previously described CO poisoning retinopathy includes disk edema, retinal vein tortuosity, vitreous hemorrhage, and retinal hemorrhage[5-8], while CO-related macular disease usually manifests itself as cystoid edema[9]. Sub-ILM hemorrhage are not common, and when they occur, they can cause severe visual damage. We examined the available literature using PubMed-Medline and found only a few reports related to CO retinopathy, none of which reported the OCTA features of macular sub-ILM hemorrhage.
In this case, SD-OCT could not distinguish the hemorrhage from the RNFL; however, FFA and ICGA confirmed that the hemorrhage was located on the surface of the macula,as it blocked all retinal and choroidal vascular fluorescence,suggesting that FFA and ICGA may help to make a clear diagnosis and the decision to perform vitrectomy.
To our knowledge, this is the first case using OCTA to observe the vascular density and microvasculature of the macular area in CO retinopathy. At the 6-month follow-up,the further deterioration of MVD in the patient’s left eye might indicate that the damage to macular capillaries in a sub-ILM hemorrhage caused by CO retinopathy is chronic and progressive; however, this requires a larger sample size and longer-term follow-up to determine.
At present, the reason for retinal vascular damage after CO poisoning was not clear. It is possible that due to severe hypoxia and ischemia caused by CO intoxication, the damage of vascular endothelial cells in the fundus leads to intima roughness, increases the aggregation and adhesiveness of platelet, and releases vasoactive substances, as well as vasospasm, the vasospasm could be transient or continuous,and induces leukocyte aggregation, adhesion, and infiltration into the intima of vessels, causing microthrombosis and thus resulting in the decrease of macular microvasculature.However, the mechanism still needs further research to confirm.
Our findings suggest that sub-ILM hemorrhage can be included as a manifestation of CO retinopathy, which can cause foveal macrovascular damage and severe vision loss and should be removed as soon as possible. In this patient, ophthalmology was not consulted until 9d after admission, when the patient complained of vision loss. Given the ocular associations of CO retinopathy, ophthalmologic consultation and vitrectomy should be considered in patients with CO poisoning.
ACKNOWLEDGEMENTS
Authors’ contributions:Zhang HD performed the operation,and was involved with data acquisition, and major contributor in the writing manuscript, Zhang L, analyzed the data, revised and edited this manuscript; Han F managed the patient, assisted the operation and was involved with data interpretation;Jiang W contributed with the interpretation of the data; Lan F contributed with the acquisition of the data.
Conflicts of Interest:Zhang HD,None;Zhang L,None;Han F,None;Jiang W,None;Lan F,None.
 International Journal of Ophthalmology2022年11期
International Journal of Ophthalmology2022年11期
- International Journal of Ophthalmology的其它文章
- Paracentral acute middle maculopathy after diagnostic cerebral angiography in a patient with a contralateral carotid-cavernous fistula
- Choroidal folds associated with carotid cavernous fistula:a case report
- Role of the ultra-wide-field imaging system in the diagnosis of pigmented paravenous chorioretinal atrophy
- A rare combination of late-onset post-LASlK keratectasia after early-onset central toxic keratopathy
- Gastrointestinal microbiome and primary Sj?gren’s syndrome: a review of the literature and conclusions
- lnsights on the possibility of SARS-CoV-2 transmission through the eyes
