Glial cells in intracerebral transplantation for Parkinson's disease
Nikola Tomov
Institute of Anatomy, University of Bern, Baltzerstrasse 2, 3012 Bern, Switzerland
Abstract In the last few decades, intracerebral transplantation has grown from a dubious neuroscientific topic to a plausible modality for treatment of neurological disorders. The possibility for cell replacement opens a new field of perspectives in the therapy of neurodegenerative disorders, ischemia, and neurotrauma, with the most lessons learned from intracerebral transplantation in Parkinson's disease. Multiple animal studies and a few small-scale clinical trials have proven the concept of intracerebral grafting, but still have to provide a uniform and highly efficient approach to the procedure, suitable for clinical application. The success of intracerebral transplantation is highly dependent on the integration of the grafted cells with the host brain.In this process, glial cells are clearly more than passive bystanders. They provide transplanted cells with mechanical support, trophics, mediate synapse formation, and participate in graft vascularization. At the same time, glial cells mediate scarring, graft rejection, and neuroinflammation, which can be detrimental.We can use this information to try to understand the mechanisms behind the glial reaction to intracerebral transplantation. Recognizing and utilizing glial reactivity can move translational research forward and provide an insight not only to post-transplantation events but also to mechanisms of neuronal death and degeneration. Knowledge about glial reactivity to transplanted cells could also be a key for optimization of transplantation protocols, which ultimately should contribute to greater patient benefit.
Key Words: astroglia; dopaminergic; glial scarring; microglia; neuroinflammation; Parkinson's disease;transplantation
Introduction
The concept of neural transplantation is not new. The very first report of transplantation of neural tissue to the central nervous system (CNS) dates back to the end of the 19thcentury (Thompson, 1890) and does include an account of what is surely a glial reaction. The “organized connective tissue”on the interface between grafted tissue and host brain was,most likely, fibrin deposit, combined with some glial scar tissue. It was firstly recognized and described as a glial layer some 15 years later (Saltykow, 1905), however, without any comments regarding its origin and functional importance.In the years to come, only occasional reports of intracerebral transplantation have been published, with scarce descriptions of glia, such as the observation of glial cell proliferation in grafted tissue by Le Gros Clark (1940).
Gopal Das (Bj?rklund, 1999) has given the first modern and scientifically sound description of the events following intracerebral transplantation. By demonstrating cell migration from the graft towards the host brain (Das and Altman,1971, 1972; Das et al., 1973), it was shown that the graft-host interface is not a static barrier, but rather a dynamic structure and site of intensive cell-cell interaction. Shortly thereafter, a functional integration of transplanted neural cells and host brain (Bj?rklund and Stenevi, 1979; Perlow et al., 1979)was demonstrated. An account of glial reaction has been provided in those seminal works - a glial layer, mediating graft adherence to the host brain tissue has been described(Bj?rklund and Stenevi, 1979). Another observation of interplay between grafted cells and host glia has been made by Perlow et al. (1979), noting growth of ependymal cells parallel to axons, growing out of the graft. After those seminal reports, neural transplantation was seen as a possible modality for cell replacement in neurodegenerative disease, which has led to an expansion of experiments in different settings, utilizing different protocols. However, the focus of those studies has almost exclusively been on the neuronal interactions,while glial cells have been more modestly commented.
Probably the most abundant body of information regarding intracerebral transplantation exists in the context of dopaminergic grafting in Parkinson's disease (PD), gathered both in animal models, as well as in some clinical trials.Despite the concept of replacing a certain degenerated cell population (in that case dopaminergic neurons) with the intent of reversing the deficit provoked by their degeneration is very straightforward, the practical results are often not so consistent. What is known both from animal studies as well as from the limited clinical experience is that intracerebral transplantation does not always work as intended. Although the procedure is generally considered safe, its efficacy is variable, and side effects are often observed (Wenker and Pitossi,2019). Multiple factors, which may influence the outcome,have been discussed, including the choice of cells for transplantation, the preparation of the material for grafting, the procedure itself, and the condition of the host brain (Collier et al., 2019). The role of glia following grafting has been marginally discussed, mostly in the context of immune reaction following grafting. The present review aims to draw attention towards the somewhat disregarded role of glial cells in intracerebral transplantation. This can attempt to elucidate the complex post-transplantational processes and give a perspective towards optimization of transplantation protocols.
Search Strategy and Selection Criteria
The articles cited in the present review were retrieved from a search in the NCBI PubMed database using “intracerebral transplantation” as keyword. Results were manually screened so relevant works containing information about astroglial and microglial reaction to transplantation were included.
Astrocytes and Transplantation
Any injury to the CNS leads to a gliotic reaction. Astrocytes around the lesion act to seal off the brain from the tissue defect. They vigorously proliferate and produce many interwoven processes, which come in contact with one another. In the uninjured CNS, astroglial cells normally produce a plentiful extracellular matrix. Upon activation by trauma, some of its components get specifically upregulated in traumatic areas only. Some of those components, such as tenascin, are inhibitory for axon growth, while others such as laminin are permissive. Furthermore, the astrocytes modulate their environment, by secreting a wide spectrum of molecules, and many of them are also neurotrophic (Barker et al., 1996).
In general, reactive astrogliosis has been schematically categorized into isomorphic (neuroprotective) and anisomorphic (scar forming). Both types may appear simultaneously at different sites following a single lesion (Ferrer, 2017), and grafting to the CNS is one of those instances. Intracerebral transplantation causes an intensive astroglial activation,which is caused by both the inevitable trauma caused by the surgery (anisomorphic), as well as the interaction between grafted cells and host brain (isomorphic).
When the neural tissue is injured, astrocytes principally act to seal off the damaged territories. By producing a glial scar, they isolate lesioned areas of the CNS, while simultaneously supporting the regeneration. Reactive astrogliosis is an extremely heterogeneous process, which can be caused by different stimuli, is mediated by different factors, has distinct functions, and leads to a multitude of, sometimes even opposite, effects (Ferrer, 2017). Extensive scarring is known to inhibit axonal growth, imposing a major barrier to regeneration. At the same time, astrocytes provide crucial trophic support at the injury site (Rolls et al., 2009). In grafting, the transplanted neural cells are challenged twofold. For successful transplantation, firstly, they have to survive the severe conditions surrounding the grafting itself, and secondly, they have to achieve integration with the host brain, and in these tasks, they encounter the two faces of the astroglial scar.
The relationship between the degree of trauma during transplantation and the percentage of transplanted cells,which integrate with the host brain, is well studied. Using a more traumatic approach leads to stronger reactive astrogliosis and to poorer survival of transplanted neurons (Nikkhah et al., 1994). In this situation, it is not clear if the correlation between the extent of astrogliosis and the number of surviving transplanted cells is directly casual. Vast glial scarring,however, could be considered rather detrimental, being a difficultly penetrable barrier for neurites, interfering with the ultimate goal of reinnervation (Silver and Miller, 2004).This has led to refining of surgical approaches; the way the cells are delivered to the host brain is still subject of ongoing research (Barker et al., 2019). Despite attempts to reduce trauma, it should be noted that the tissue disruption caused by the surgical approach is an unevitable event, since the blood-brain barrier is breached at the moment of the insertion of even the most elegant grafting instrument. Therefore,trauma-induced astroglial activation will always be present following transplantation.
However, what is observable as extensive astroglial recruitment around grafts, is not per se a glial scar, and is not automatically deleterious. A recent study (Tomov et al., 2018)shows that grafted cells apparently actively induce astrogliosis surrounding the graft. This astrogliosis can be categorized under the already discussed isomorphic type. Evidence from this study suggests that astrocytes produce a glial scaffold for the grafted dopaminergic cells. By orienting their processes parallel to the axons growing out from the transplanted neurons (Isacson et al., 1995, Mendez et al., 2005), astroglia of the host brain actually actively aid the reinnervation of the dopamine-depleted striatum. Furthermore, it is known that following grafting, some afferent projections from the host brain can also reach the transplant (Petit et al., 2001). In both cases, the astroglial envelope around the graft is a penetrable barrier for neurites (Li et al., 2012), among others because it contains less extracellular matrix, which is preconditioned by the presence of transplanted cells (Barker et al., 1996).
Consistent with the idea of the tripartite synapse (i.e.,presynapse, postsynapse, and astrocyte), the astroglia of the host striatum plays a crucial role in the synaptic integration of the grafted cells. It is widely known that astrocytes associated with synapses exchange information with neurons, with implications for sustaining synaptic strength. In the context of PD, data shows that there is a significant increase of astrocytic presence in striatal tripartite synapses in the parkinsonian brain (Villaba and Smith, 2011). This further highlights the role of astrocytes in restoring the neuronal circuitry following grafting and gives a good explanation of the observed intensive astroglial recruitment around grafts.
Another beneficial aspect of astroglial activation following transplantation is providing trophics for the grafted tissue.The astrocytes of the host brain could be activated early, immediately after surgery, and could directly provide glucose for the transplanted cells via glycogenolysis (Forno et al.,1992), thereby reducing the metabolic damage caused by the preparation of the material for grafting. In the longer term,astrocytes also mediate the vascularization of grafted tissue.The commonly used protocol for preparation of a single cell suspension (Pruszak et al., 2009) yields a completely avascular graft. Therefore, the formation on a vascular bed to the graft is a critical stage in its integration. The trauma from the transplantation itself can be viewed as a major vasculogenic stimulus in the CNS (Ment et al., 1997), with astrocytes playing a key role in the formation of the basal lamina of the newly formed vascular endothelium (Lawrence et al., 1984),also interacting with pericytes (Silver and Miller, 2004). On a morphological level this is best demonstrated by the dense astroglial envelope around graft-associated blood vessels(Tomov et al., 2018). The perivascular astrocytic elements are predominantly derived from astrocytes of the host striatum,activated by the transplantation, with lesser numbers being derived from cells in the grafted suspension (Krum and Rosenstein, 1989). This once again speaks for the activation of host astrocytes by grafted cells, promoting organotypic graftdevelopment and integration.
Recently, it has been suggested that transplantation of neural stem cells in a model of PD leads to astrocyte-dependent activation via the canonical Wnt pathway. Ultimately, this leads to activation of neurotrophic and anti-inflammatory/anti-oxidant mechanisms, which reduce neuroinflammation and initiate a neurorestorative program for dopaminergic neurons (L'Episcopo et al., 2018). This suggests that as-trocytes might mediate indirect effects of transplantation,which generally improves the metabolic state of the CNS,implicates a great, previously unexplored potential of cell-replacement therapies, not only for PD, but also for other neurological disorders.
Evidence suggests that astrocytes are key players in the restoration of the dopaminergic circuitry after grafting in PD. The intensive astrogliosis, surrounding grafts, which can resemble scarring, is in fact isomorphic, and is extremely important for the integration of the transplanted cells. The stimulation by the graft, facilitating axonal outgrowth, synaptic formation, and vascularization, greatly outweighs the activation by the mechanical tissue disruption. Moreover,astrocytes can mediate neuroprotective, neurotrophic, and immunomodulatory effects beneficial for endogenous dopaminergic neurons, by mechanisms not directly involved with reinnervation by the graft
Microglia and Transplantation
Following any mechanical intervention to the CNS, microglia rapidly engage in the neuroinflammatory response. Upon functional activation, they undergo morphological changes and get involved in phagocytosis, respectively antigen presentation, the production and secretion of reactive oxygen species, multitude of cytokines and growth factors (Appel et al., 2010). Grafting to the CNS also inevitably causes activation of the host's microglia. Like in the case with astroglia,we consider this to be a two-staged process, involving both the tissue trauma from the transplantation itself, as well as the immune reaction against the grafted cells, the latter involving both the innate and the adaptive immunity (Tomov et al., 2019).
Microglial cells are rapidly activated upon disruption of neural tissue; they migrate towards the broken glia limitans,project their processes and act together with astrocytes to restore its integrity (Corps et al., 2015). Experimental data shows that pharmacological blocking of microglial reactivity leads to reduced migration, and subsequently to exacerbation of the tissue injury (Nimmerjahn et al., 2005; Davalos et al., 2005; Haynes et al., 2006; Koizumi et al., 2007). Despite this, the consensus is that acute microglial response is generaly neuroprotective (Roth et al., 2004), while prolonged microglial activation is considered maladaptive (Zhang et al.,2014). Therefore, when discussing the role of microglial cells in post-transplantation events, attention should be given to the mode of microglial activation.
Different activation status of microglial cells can promote either neurotoxicity or neuroprotection (Appel et al., 2010).The classically activated M1 microglial cells secrete pro-inflammatory cytokines and reactive oxygen species, thereby being neurotoxic. At the same time, the alternatively activated M2 microglial cells assist in inflammation resolution by neurotrophic factor release (Michelucci et al., 2009). Very interesting in this context is the cross talk between microglia and T lymphocytes. It is a regulatory mechanism, which can trigger either the classical or the alternative activation pathway of microglial activation, thereby switching the reaction towards a less inflammatory and neuroprotective profile (Appel et al., 2010). In PD, the activation of the microglia classically follows the M1 phenotype, ultimately leading to the cell death of the microglia-surrounded dopaminergic neurons(McGeer et al., 1988; Hald and Lotharius, 2005). The process is associated with significant immune cell activation in the substantia nigra and along the nigrostriatal pathway (McGeer et al., 1988; Appel et al., 2010). It is not certain if exactly the same immunological processes affecting endogenous neurons in PD can also affect the transplanted neurons in cell replacement therapy. It has been proposed that Lewy body pathology, which was observed in grafts in a PD model, is a reaction to inflammation at the graft-host interface, and is mediated by microglia (Gao et al., 2008, George et al., 2019).
We consider the mechanisms involved in neurodegeneration in PD to be also involved in the observed massive degeneration of transplanted dopaminergic neurons (Barker et al., 1996), and at least some of them to be microglia-mediated. Following transplantation, microglial activation is dependent on immunological compatibility between graft and host, the way of delivery of transplantation material, the presence or absence of anti-inflammatory therapy, and the degree of synaptic integration between graft and host.
It has been suggested, that there are two mechanisms of microglial recruitment following grafting (Tomov et al.,2019). The first one is a migration of cells of bone marrow origin to the brain (Ginhoux and Prinz, 2015), and the second is the proliferation of resident microglia. The reported increased permeability of graft-associated blood vessels for several days after transplantation (Akalan and Grady, 1994)is a prerequisite for extravasation of blood-borne cells, which transform into microglia. However, the contribution of such cells to the microglial population of the adult CNS is relatively small. The main mechanism of microglial recruitment is the proliferation of brain microglia (Ginhoux and Prinz,2015), i.e. the majority of microglial cells surrounding grafts are indeed progeny of resident cells.
Microglial cells have been described infiltrating the transplants as soon as 3 days after grafting (Wenker and Pitossi,2019). Microglial cells with activated (ameboid) appearance surround grafts and infiltrate the graft core. The microglial infiltration persists for a very significant amount of time post-grafting (Shinoda et al., 1996).
The data regarding the duration of acute microglial activation following grafting is not unambiguous, with reactive microglia persisting for years in cases of patients receiving a graft (Olanow et al., 2003). The notion that acute events should account for retaining the activated phenotype of microglia for only about 10 days (Harry and Kraft, 2012)suggests that grafting accounts for a sustained, prolonged microglial activation.
The degree of microglial infiltration directly correlates with the degree of rejection (Kelly et al., 2004). However,merely the ameboid appearance, being suggestive for an activated phenotype of microglia, does not correlate with expression of CD68, which would hint towards phagocytic activation (Li et al., 2008). The presence of activated microglia around and within grafts, therefore, is not directly equal to processes of graft rejection and is normally seen in “healthy”grafts (Kordower et al., 1997). This microglia should be classified as M2-activated. It is clear though that e.g. immunological incompatibility between donor and recipient is likely to induce a rejection, manifested as a long-lasting inflammatory response accompanied by M1 activation of microglia and macrophages.
For a long time, the brain had been considered to be an organ out of the vigilance of the immune system. Therefore, it has been assumed, that cell transplants into the brain would not need immonsuppressive therapy. However, experiments have shown that the CNS is not absolutely immunopriviledged, and transplants to the brain are indeed immunogenic(Barker and Widner, 2004). Clinical evidence clearly demonstrates that in allogeneic transplantation, immunosuppression is necessary, in order to achieve survival of grafts (for a review see Winkler et al., 2005). Moreover, immunological provocation has been strongly associated to graft rejection(Piquet et al., 2012). This indicates that activated microglial cells observed after grafting are involved in an ongoing immune-mediated inflammatory process.
A major activator for microglia could be the donor blood vessels expressing high levels of the major histocompatibility complex (MHC) class I molecules (Finsen et al., 1991). The preparation of suspension grafts usually destroys those vascular structures, shifting the equilibrum towards host-driven angiogenesis (Mendez et al., 2005; Cooper et al., 2009) and at the same time fundamentally reducing immunogenicity.In those instances, the most intensive microglial reaction remains along the needle tract (Mendez et al., 2005), probably due to the tissue trauma itself. The reports of MHC class II upregulation and intensive microglial response to transplants of solid tissue pieces in Parkinson's disease patients (Kordower et al., 1997; Freed et al., 2001) also suggest that preparation of a single-cell suspension is a good strategy to evade a major microglia-driven neuroinflammation.Thereby, the amount of non-neural elements in the material to be transplanted should be maximally reduced. In this direction, it has been proposed that MHC graft-host matching along with immunosuppression is the best strategy to evade immune rejection of intracerebral grafts. This strategy significantly reduces, but does not completely neutralize the microglial reaction (Morizane et al., 2017). Activation of microglia can be attributed to the existence of MHC-independent antigens (Mizukami et al., 2014). However, the great complexity of events around the intracerebral graft leaves room for other non-immunological mechanisms of microglial activation, such as synaptic formation, which will be discussed later in the present work.
Intensive microglial recruitment along the graft-host interface is known to correlate with worse functional outcome,despite not directly being associated with graft rejection(Winkler et al., 2005). Evidence points towards the notion that excessive microglial activation leads to aberrant synaptic formation, associated with the development of graft-induced diskynesias (Soderstrom et al., 2005) — a much-feared complication in the clinical application of cell therapy. Pro-inflammatory cytokines have been associated with a gene expression pattern, consistent with activation (Kyriakis and Avruch, 1999), such as the upregulation of FosB/ΔFosB, a transcription factor upregulated in animal models of dyskinesias (Cenci, 2002; Maries et al., 2006). Therefore, the participation of microglia in the development of graft-induced dyskinesias remains an open topic to provide transplant recipients with good quality of life.
In one of the clinical studies with intracerebral dopaminergic transplantation, patients with seemingly functional grafts deteriorated quickly after withdrawal of immunosuppression, with a postmortem finding of extensive microglial infiltration of the grafts (Olanow et al., 2003). Moreover, the expression level of general markers of immune response has been correlated with the degree of deterioration of grafts with poorer functional outcome (Kordower et al., 2008).Therefore, it is certain that successful engraftment of (allogeneic) dopaminergic grafts without systemic immunosuppression is extremely difficult to obtain.
Despite the obvious benefits, continuous immunosuppression in a clinical setting leads to considerable morbidity (Piquet et al., 2012). At the same time, it is known that dopaminergic neurons may be beneficed and protected by anti-inflammatory intervention targeting microglia (Barcia et al., 2011). This has sparkled interest towards more specific glia-oriented intervention for enhancing results of dopaminergic transplantation and has shown that selective microglial inhibition is possible (Tomov et al., 2019). While in xenotransplantation, inhibition of microglia is generally beneficial (Michel-Monigadon et al., 2010), the results of Tomov et al. did not show a relationship between extent of microglial recruitment and the number of dopaminergic neurons integrating after transplantation. In this perspective,a longer follow-up of the results of modification of glial reactivity is needed in order to confirm potential applications for enhancing functionality of grafts.
A great body of information clearly shows that microglia is not just “the bad guy” in neuroinflammation. It is known that the activation profile of microglia can be switched from the neurotoxic M1 to the neuroprotective M2 phenotype via several mechanisms, including cytokine-mediated interaction and direct influence by CD4+regulatory lymphocytes(Comi and Tondo, 2017). This phenomenon has an utter importance for the survival and integration of the graft. Axonal regeneration is known to involve microglial cells (Shokouhi et al., 2010). Furthermore, evidence supports a role for microglia-secreted inflammatory mediators in synaptic plasticity (Leonardo, 2005). Such cytokines can even lead to increased synaptic strength (Bains and Oliet, 2007).
The importance of microglia for forming and maintaining synaptic connections is widely known (Trapp et al., 2007;Wake et al., 2009). The interaction between neurons and microglial cells is bidirectional, and the activity of neurons can directly activate microglia (Hirrlinger et al., 2004; Nimmerjahn et al., 2005; Hung et al., 2010). Activated microglia actively eliminates structures from weakly active synapses(Stellwagen and Malenka, 2006), but is also involved in regeneration of interrupted neural fibres (Prewitt et al.,1997). Dopaminergic neurites, growing beyond the area of a mechanical lesion, are intimately associated with activated macrophages (Batchelor et al., 1999). Those fact suggest that reinnervation of the host brain by the dopaminergic graft is closely related to microglial activity. The observed prolonged microglial activation, persisting for many weeks following grafting (Barker et al., 1996; Stott and Barker, 2013; Tomov et al., 2019) is an indirect evidence for the synaptic integration of the grafted tissue.
Despite we have attempted to give separate accounts of the astroglial and microglial activation, one should keep in mind that the mechanisms of activation of both cell populations are similar. Both astrocytes as well as microglial cells express receptors for the same proinflammatory cytokines (Barcia et al., 2011). Therefore, we can conclude that neuroinflammation itself is also a major stimulus for reactive astrogliosis and perpetuates the glial activation surrounding the graft.The balance between isomorphic astrogliosis, driven by M2 activation of microglia, and glial scarring, caused by microglia with M1 phenotype and the exact molecular mechanisms affecting it is still a topic of discussion.
What Do We Know and Where Are We Going?
Neuropathological data suggests that persistent glial activation in PD may be responsible for perpetuating neuroinflammation and contributing to neuronal degeneration (Barcia et al., 2011). Abundant data also suggests that anti-inflammatory therapy may be beneficial in PD patients (Chen et al., 2005). This can lead us to the conclusion that the mechanisms, involved in glial activation in sporadic PD and following intracerebral transplantation could lead to the same detrimental effects for the dopaminergic neurons. Given that anti-inflammatory therapy elegantly targeting only selected crucial mechanisms might be a promising therapy for many CNS conditions, we believe that manipulating the glial response following intracerebral transplantation is relevant for the applied cell therapy of PD.
Pioneer of dopaminergic transplantation Ole Isacson said “The cell that you would like to transplant is the fetal A9 neuron with appropriate glial support, but we don't yet have that” (Isacson et al., 2003). Recent advancements from the field of induced pluripotent stem cell research (Stoddard-Bennett and Reijo Pera, 2019) might soon solve the problem with the cell to be transplanted. This gives hope to raise the self-imposed moratorium over the dopaminergic cell transplantation in patients. How exactly a transplantation is “correctly performed”, is still a subject of debate,which should take multiple factors into consideration (Collier et al., 2019). We, as Ole Isacson, suggest that attention should be paid to the glial cells as well, as important players in post-grafting events. Understanding specific mechanisms of glial activation as well of glia-graft interaction means understanding one of the multiple factors, which affect the results of intracerebral transplantation in PD. Only by optimizing the patient's chance to receive benefit from this procedure we can hope to move past the experimental into the applied setting.
Conclusion
Experimental data shows that the adult brain is a very plastic system, capable of incorporating transplanted neurons into functional systems. After transplantation, host glial cells exert multiple effects, both beneficial and detrimental, as outlined in Figure 1. Elucidating the molecular mechanisms of glia-graft interaction should provide clues for developing more effective cell-replacement therapies for the future and lead to better functional results for patients.
Author contributions:The author completed the work independently.
Conflicts of interest:The author declares no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
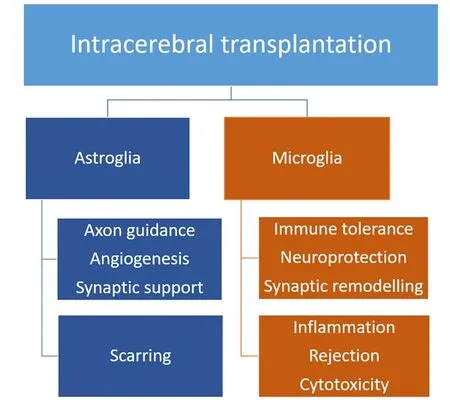
Figure 1 A schematic outline of beneficial and detrimental effects of glial activation following intracerebral transplantation.
Open peer reviewers:Agustin Cota-Coronado, Medical and Pharmaceutical Biotechnology, Mexico; Cristoforo Comi, University of Piemonte Orientale, Italy.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Fast-tracking regenerative medicine for traumatic brain injury
- The N-formyl peptide receptors: contemporary roles in neuronal function and dysfunction
- Adrenomedullin: an important participant in neurological diseases
- Shifting equilibriums in Alzheimer's disease: the complex roles of microglia in neuroinflammation,neuronal survival and neurogenesis
- ABC efflux transporters at blood-central nervous system barriers and their implications for treating spinal cord disorders
- Biomaterials and neural regeneration