ABC efflux transporters at blood-central nervous system barriers and their implications for treating spinal cord disorders
Liam M. Koehn
Department of Pharmacology and Therapeutics, the University of Melbourne, Parkville, Victoria, Australia
Abstract The barriers present in the interfaces between the blood and the central nervous system form a major hurdle for the pharmacological treatment of central nervous system injuries and diseases. The family of ATP-binding cassette (ABC) transporters has been widely studied regarding efflux of medications at blood-central nervous system barriers. These efflux transporters include P-glycoprotein (abcb1), ‘breast cancer resistance protein' (abcg2) and the various ‘multidrug resistance-associated proteins' (abccs). Understanding which efflux transporters are present at the blood-spinal cord, blood-cerebrospinal fluid and cerebrospinal fluid-spinal cord barriers is necessary to determine their involvement in limiting drug transfer from blood to the spinal cord tissue. Recent developments in the blood-brain barrier field have shown that barrier systems are dynamic and the profile of barrier defenses can alter due to conditions such as age, disease and environmental challenge. This means that a true understanding of ABC efflux transporter expression and localization should not be one static value but instead a range that represents the complex patient subpopulations that exist. In the present review, the blood-central nervous system barrier literature is discussed with a focus on the impact of ABC efflux transporters on: (i) protecting the spinal cord from adverse effects of systemically directed drugs, and (ii) limiting centrally directed drugs from accessing their active sites within the spinal cord.
Key Words: ABC transporters; ATP-binding cassette; BCRP; blood-brain barrier; blood-spinal cord barrier;efflux; MRP; P-glycoprotein; PGP; spinal cord injury
Introduction
Spinal cord injuries and diseases are often debilitating for patients and require diverse medical interventions. Drugs are applied to treat a range of peripheral symptoms associated with spinal conditions including urinary tract infections,cardiac dysfunction and maintenance of blood pressure (Hagen et al., 2011). Medical research is also currently working on developing spinal cord-directed medications that help to prevent tissue loss, preserve/regenerate spinal function or treat individual symptoms (Varma et al., 2013; Fakhoury,2015; Koehn et al., 2016). Recent developments in the bloodbrain barrier field have described a series of efflux transporters that limit the transfer of potentially beneficial medications into the central nervous system (CNS) and describe their variable localization and function for different patient populations (Bauer et al., 2004; Nies et al., 2004; Tachikawa et al., 2005; Daood et al., 2008; Gazzin et al., 2008; Virgintino et al., 2008; Cui et al., 2009; Daneman et al., 2010a; Ek et al., 2010; Kratzer et al., 2013; Hoque et al., 2015; M?llg?rd et al., 2017; Koehn et al., 2019). This information about molecular restriction into the CNS provided by efflux transporters is integral for the spinal cord injury field due to the host of drugs used in spinal conditions. Medical practitioners need to be acutely aware of the likelihood of peripherally directed treatments accessing the vulnerable spinal cord of these patients where toxic side effects may be more pronounced than in the healthy population. Drug developers and scientific researchers also need to be aware of which compounds are more or less likely to access the spinal cord when designing treatments intended to access the CNS from the systemic circulation. In the present review, the barriers between blood and CNS will be described with a focus on how their efflux capacity may relate to spinal cord health both in terms of protection from potentially toxic compounds in the periphery and limiting the transfer of potentially beneficial medical agents.
Literature within this review was identified on the NCBI platforms PubMed and Gene Expression Omnibus and the Google Scholar platform prior to August 2019 and contain research regarding ABC efflux transporter expression at barriers within the central nervous system.
Molecular Transport from Blood to Central Nervous System
The barrier systems that separate the blood and the CNS have been areas of long-standing interest. Lewandowsky(1900) conducted some of the original experiments in this field when he applied compounds such as sodium ferrocyanide to a range of species either systemically or via the subarachnoid space of the spinal cord. He noted a greater toxicity when the toxins were applied directly to the cerebrospinal fluid (CSF) prompting one of the first claims that CNS barriers may hinder the transit of certain compounds from the blood (Lewandowsky, 1900). Goldmann (1909, 1913)built on this idea, applying Trypan blue (960Da) into a rat either via the periphery or into the subarachnoid space of the lumbar spinal cord, which resulted in staining that showed the dye would not cross the CNS barriers in either direction.These experiments, along with a host of others, initiated the ever-growing field of research regarding the transfer of molecules between the systemic circulation and the CNS. They also indicate the dual nature of these barriers: preventing toxic compounds in the periphery from causing damage to the CNS while also limiting the access of any potentially beneficial medicines applied systemically from accessing the CNS.
As outlined in Figure 1, there are a series of different barriers that prevent molecular transfer from the blood to the CNS. These include the vessels that form a direct barrier between the blood and both the brain (blood-brain barrier,BBB) and the spinal cord (blood-spinal cord barrier, BSCB).In addition there are the barriers that separate blood and the CSF at the choroid plexuses (blood-CSF barrier), the pial blood vessels of the meninges and the arachnoid layer of the meninges. Finally there are the series of interfaces between the CSF and the CNS such as the ventricular neuroepithelium/ependyma, the central canal of the spinal cord and the pial/basement membrane/glial separation between the subarachnoid CSF and the brain or spinal cord. These barriers are not only different to one another in terms of functional capacity to prevent molecular transfer but each individual barrier is known to be variable between different species,ages and environmental conditions.
Protective Mechanisms at Blood-Central Nervous System Barriers
There are two main classes of protective mechanisms to prevent molecular transfer between the blood and the CNS.Water-soluble molecules are prevented from paracellular transfer by morphological barriers based on tight junctions that hold the cells of the barrier together (M?llg?rd and Saunders, 1975; Madara, 1998; Zhao et al., 2015). In the vessels of the spinal cord prototypical tight junction proteins have been shown to be present, such as ZO-1, claudin-5 and occludin, with disease models associating a decrease in these proteins with increased permeability of the BSCB (Zhong et al., 2008).
Most common medications are small molecules with high lipid solubility. Lipid-soluble molecules are able to diffuse through the lipid bilayer membrane of the barrier cells themselves and pass through to the other side via intracellular transfer. A second series of barrier defenses are present to limit the transfer of unwanted, potentially harmful lipid soluble molecules from accessing the CNS. Lining the membranes of barrier cells is a series of transporters that, among other functions, limit molecular transfer by exporting their substrates back into their original compartment (Staud et al., 2012; Nigam, 2015). A host of transporters have been linked to molecular exclusion of drugs and toxins at blood-CNS barriers, including members of the solute carrier family such as the organic anion transporting polypeptides (OATPs;Gao et al., 1999; Asaba et al., 2000). The transporter family that has had the strongest association with limiting drug permeability from the systemic circulation to the CNS is the ATP-binding cassette (ABC) transporter family of proteins.Throughout this review, ABC transporters will be described as both the protein (e.g., P-glycoprotein; PGP) and gene name (e.g.,abcb1a), with the term most accurately representing the study performed listed first and the secondary term in brackets. Human genes are listed as capitals, with animal studies in lower case.
The ABC transporter family consists of 48 genes (for humans) spanning the gene codesabcatoabcg(Chen and Tiwari, 2011). The most widely researched of these are P-Glycoprotein or PGP (abcb1;MDR1), ‘breast cancer resistance protein' or BCRP (abcg2) and the ‘multi-drug resistance-associated proteins' or MRPs (abccs). These transporters are known to exist at barriers between the blood and the CNS(Nies et al., 2004; Tachikawa et al., 2005; Daood et al., 2008;Gazzin et al., 2008; Virgintino et al., 2008; Daneman et al.,2010a; Ek et al., 2010; M?llg?rd et al., 2017) as well as between the blood and the CSF (Tachikawa et al., 2005; Daood et al., 2008; Ek et al., 2010; Kratzer et al., 2013; M?llg?rd et al., 2017) and the CSF and the CNS (Daood et al., 2008;M?llg?rd et al., 2017; Koehn et al., 2019). Drugs and molecules are able to access the spinal cord from the blood directly or via the CSF. In order to understand the likelihood of a drug accessing the spinal cord we must understand which ABC efflux transporters are present on the BSCB (spinal vascular endothelial cells), the range of blood-CSF barriers and the barriers between that CSF and the spinal cord (Figure 1).
ATP-Binding Cassette Efflux Transporters at the Spinal Cord Barriers
The blood-spinal cord barrier
The spinal cord is highly vascularized, with a complex network of endothelial cell-lined vessels projecting throughout the spinal tissue (Miyasaka et al., 2000). The walls of these blood vessels form both a physical and functional barrier interface between the systemic circulation and the spinal cord.Studies have identified ABC efflux transporter gene expression and their protein-product localization in spinal cord tissue and isolated spinal cord vessels, as well as some studies investigating the functional capacity of the transporters at this barrier (see below).
Nishimura and Naito (2005) analyzed 49 human spinal cord samples (aged 15-66 years) using RT-qPCR and found thatABCA2was the highest expressed ABC efflux transporter in the spinal cord, over an order of magnitude higher than the next highest expressed transporter (Nishimura and Naito, 2005).ABCA2was expressed over 4 fold higher in the spinal cord than in the other 22 tissues studied (Nishimura and Naito, 2005). RNAseq data on rat spinal cord homogenate not only also showed thatabca2is the highest expressed ABC efflux transporter in the rat spinal cord but the expression was in the top 1% of all genes in the tissue (Koehn et al.,2016). Studies have localizedabca2to oligodendrocyte cells in the spinal cord, with knockout models showing abnormal myelination and spinal function (Zhou et al., 2002; Mack et al., 2007). While cell line studies have suggested a link betweenABCA2and drug acquired resistance to compounds such as mitoxantrone (Boonstra et al., 2004) and estramustine (Laing et al., 1998), there is yet to be any major link established between expression of this transporter and activity at the BSCB established.
The most widely studied ABC efflux transporters at blood-CNS interfaces in relation to drug efflux are PGP (abcb1),BCRP (abcg2) and MRP1-5 (abcc1-5; Ek et al., 2010; Koehn et al., 2019). McCallum-Loudeac et al. (2019) conducted an RNAseq study analyzing the spinal cord of 10 mice(four 4-week old, six 8-week old; even male/females). The resulting data indicated thatabcc5(MRP5) was the highest expressed transporter, in excess of 6 fold higher thanabcc1(MRP1),abcb1a(PGP),abcc4(MRP4) andabcg2(BCRP).These transporters were expressed 2-4 fold higher than the transportersabcc3(MRP3) and theabcb1bisoform (PGP),both of which were 5-8 fold higher in expression thanabcc2(MRP2), which appears to be minimally expressed (if at all)in the spinal cord of the mouse (McCallum-Loudeac et al.,2019). RNAseq analysis in the rat has revealed similar results, withabcc5(MRP5),abcb1a(PGP) andabcg2(BCRP)expressed to the highest extent in spinal cord homogenate,withabcc4(MRP4) expressed at half the levels of the major transporters andabcc3(MRP3) expressed another 2 fold lower (Koehn et al., 2016). Once againabcc2(MRP2) had extremely low expression (Koehn et al., 2016).
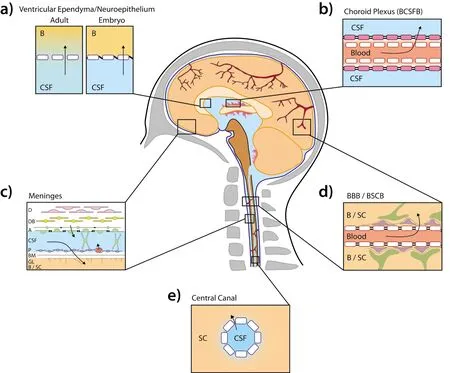
Figure 1 Interfaces between the blood and the central nervous system (CNS).The barriers/interfaces that separate molecular transport from the systemic circulation and the CNS are summarized, in the context of the human. a)The interface in the ventricle that separates molecules in the cerebrospinal fluid (CSF) and the brain. This interface is termed the ependyma in the adult and has ventricular ependymal barrier cells (blue outline, white fill) separated by gap junctions allowing unhindered paracellular exchange. In the developing fetus the cells of this barrier, termed the neuroepithelium, are held together tightly by ‘strap junctions' limiting paracellular transfer allowing molecules to access the brain by transcellular transport. b) The barrier between the circulation and the CSF at the choroid plexus epithelium (BCSFB). The endothelial cells of blood vessels (red outline, white fill) are largely fenestrated with epithelial cells of the choroid plexus (pink fill) held together by tight junctions preventing paracellular transfer. c). The layer of meninges that surrounds both the brain (B) and spinal cord (SC).This contains the dura (D), dural boarder cells (DB), arachnoid cells (A), pial cells (P), basement membrane (BM) and glial limitans (GL). Tight junctions exist in the arachnoid cell layer preventing paracellular transfer from molecules exiting the fenestrated blood vessels in the dural layers from accessing the arachnoid space CSF. Tight junctions also exist at the blood vessels within the pial cell layer, preventing paracellular transfer into the arachnoid CSF. d) The barriers between the blood and brain (blood brain barrier proper; BBB) and the blood and spinal cord (blood spinal cord barrier proper, BSCB). The endothelial cells of the blood vessels (red outline, white fill) are held together by tight junctions preventing paracellular transfer. Pericytes (purple fill) and astrocytes (green fill) line the outside of the barrier on the CNS side. e) The central canal of the spinal cord with ependymal cells separating CSF from spinal tissue. Arrows indicate likely routes of molecular transfer. The present figure was adapted from previous illustrations (Ranson, 1959; Saunders et al., 2016) with morphological accuracy determined from multiple studies (Brightman and Reese, 1969;M?llg?rd and Saunders, 1975; Nabeshima et al., 1975; Bruni and Reddy, 1987; M?llg?rd et al., 1987; Vandenabeele et al., 1996; Zhong et al., 2008;Daneman et al., 2010b; Garbuzova-Davis et al., 2012; Br?chner et al., 2015; Whish et al., 2015).
Transcriptomic analysis in human spinal cord material suggests some similarities between the rodent studies and human tissue. Su et al. (2002) undertook a large-scale array study that included 2 samples of human spinal cord. In the data from their studyABCB1(PGP),ABCG2(BCRP) andABCC5(MRP5) predominated.ABCC4(MRP4),ABCC1(MRP1) andABCC3(MRP3) also had notable levels of expression.ABCC2(MRP2) had extremely low levels of detection. The human RT-qPCR study by Nishimura and Naito(2005; described above) also hadABCB1(PGP),ABCG2(BCRP) andABCC5(MRP5) with high levels of expression,along withABCC4 (MRP4) andABCC1(MRP1). All five genes were expressed within 2 fold of one another (Nishimura and Naito, 2005).ABCC3(MRP3) was expressed 2 fold lower thanABCB1(PGP), withABCC2(BCRP) expressed an order of magnitude belowABCC3(MRP3).
While the above studies provide valuable insight into ABC transporters in the spinal cord, they do not investigate the BSCB specifically. Distinct differences in the expression profile of transporters between tissue homogenate and isolated endothelial cells have been shown in the blood-brain barrier field. RNAseq analysis on brain homogenate has revealed a similar profile of ABC efflux transporter expression to the spinal cord described above:abcc5>abcc1>abcb1a>abcg2>abcc4>abcc3/abcb1b(Yu et al., 2014). A study by Zhang et al. (2014) in mice showed thatabc1a/abcb1b(PGP),abcg2(BCRP) andabcc4(MRP4) all were enriched in cerebral endothelial cells compared to neuronal and glial cells, with these genes being expressed higher thanabcc5andabcc1.Other studies have suggested thatabcb1a(PGP) in particular is highly localized to endothelial cells with over 30 fold more expression compared to the brain parenchyma (Daneman et al., 2010b). Further studies on mouse brain endothelial cells have replicated these results suggestingabcb1ais the highest expressed ABC transporter at the blood-brain barrier specifically, followed byabcg2,abcc4,abcc5and abcc1(Yousif et al., 2007).
Unlike published comparisons between cortical homogenate and isolated cerebral endothelial cells, there is yet to be a direct comparison provided between spinal tissue homogenate and spinal cord endothelial cells. There have, however,been some studies on isolated spinal endothelial cells reporting protein and function of ABC efflux transporters. Campos et al. (2012) performed western blotting on isolated capillaries from rat spinal cord revealing the presence of PGP, BCRP and MRP2 in rat spinal cord endothelia.Ex vivotransport assays of the capillaries showed functional activity of all 3 transporters on the luminal side of the barrier (Campos et al., 2012). Similar isolated spinal cord capillary studies also indicate a functional role of MRP1 at the BSCB (Cartwright et al., 2013). The presence and function of MRP2 at the barrier despite the extremely low transcriptomic expression(described above) requires further investigation. This may include a comparison of expression in spinal cord homogenate with isolated endothelial cells (where expression may be higher), as well asin vivotransfer studies for substrates of each transporter type to describe the relative functional capacity of each at the BSCB. Immunohistochemical localization of PGP to the luminal membrane of endothelial cells in spinal vessels confirms the strong evidence of its role at the BSCB (Yasuda et al., 2013). Previous literature has suggested that the levels of PGP may differ between the BBB and BSCB(Ge and Pachter, 2006), however the functional significance of this difference and whether other transporters may be expressed higher at the BSCB than the BBB is yet to be fully investigated.
From the studies described above it appears thatABCB1(PGP) andABCG2(BCRP) are likely to be the major ABC efflux transporters in the BSCB of the human and rat. High expression (particularly in rodents) was observed forABCC5(MRP5) warranting further investigation, withABCC1(MRP1) andABCC4(MRP4) also expressed highly. Other MRP transportersABCC2(MRP2) andABCC3(MRP3)have also been implicated at this barrier despite exhibiting lower levels of transcription. Future experiments to examine the other transporters with high spinal cord expression, such as members theabcafamily, would be valuable to the field.However, to apply this knowledge to future research that has translational potential it must be known whether the transporter levels described reflect those in the primary patient populations.
Dynamic regulation of ABC efflux transporters in the blood-spinal cord barrier
In the paragraphs above the expression levels of ABC efflux transporters is described in the control adult (rodent and human) spinal cord at the BSCB. The level of expression of these transporters, however, is known to be highly variable and dynamic. As described in a multitude of studies, barrier cells are able to increase and decrease the level and functional capacity of these efflux defenses when required including changes over the course of development (Daood et al., 2008;Gazzin et al., 2008; Daneman et al., 2010a; Ek et al., 2010;Kratzer et al., 2013), following different environmental challenges such as chronic medication use (Bauer et al., 2004;Cui et al., 2009; Hoque et al., 2015; Koehn et al., 2019) and in response to disease and injury (Dombrowski et al., 2002;Volk and Losher, 2005; Deo et al., 2014).
Spinal cord injuries and disorders are not isolated to one stage of development; with conditions known to arise from birth or early in life (Phillips et al., 2018), the high incidence of spinal cord injury in adolescents and young adults (Sekhon and Fehlings, 2001) as well as incidences in the elderly population (Krassioukov et al., 2003). This means that for doctors to have a good understanding of how to treat these patients (both for systemic symptoms and the injury directly), we must understand how the level of ABC efflux transporters at spinal interfaces may change with age. Studies looking at changes in ABC efflux transporter expression in the spinal cord over development are not widespread. As described above, McCallum-Loudeac et al. (2019) analyzed two post-weening ages (P28 and P56) of rats in a spinal cord homogenate using transcriptomic analysis. In their datasets there were no significant differences in ABC efflux transporter expression forabcb1a/abcb1b(PGP),abcg2(BCRP) orabcc1-5(MRP1-5) between the 4 and 8-week ages. Saunders et al. (2014) have described the expression of ABC transporters at much earlier developmental stages in theMonodelphis domestica(South American opossum).This species is born at stages of brain and choroid plexus development similar to E13-14 embryonic rodents (Saunders et al., 1989; Dziegielewska et al., 2001). In theMonodelphis domesticaspinal cord datasets at both P8 and P29 the expression ofabcc5(MRP5) andabcg2 (BCRP) predominated,with expression ofabcb1(PGP),abcc1/2/4(MRP1/2/4) present in similar amounts (trend observed in both P8 and P29).Higher expression ofabcb1(PGP; ~3 fold) andabcg2(BCRP;~2 fold) was found in the P29 pups compared to P8 (Saunders et al., 2014). This up-regulation inabcb1a(PGP) in the spinal cord over development matches that described in the brain in other studies (Daood et al., 2008; Gazzin et al., 2008;Daneman et al., 2010b; Ek et al., 2010).
Individual patients can differ greatly in terms of medications they are taking specifically for their condition and for additional ailments. The level of expression of ABC efflux transporters in the spinal cord in the adult rodent has been shown to vary following chronic drug challenge of isolated barriers. A study by Wang et al. (2014) on isolated mouse spinal capillaries showed that the transport capacity of PGP at the BSCB could be doubled if the capillaries were previously exposed to molecules such as sulforaphane, 2,3,7,8-Tetrachlorodibenzo-p-dioxin or pregnenolone 16α-carbonitrile.These results suggest that if such a directed drug is taken either alongside another medication or alone over a period of time the access of that drug to its active site in the spinal cord may decrease as BSCB increase transport functionality in response to drug challenge. Studies expanding these results to in anin vivosetting analyzing the chronic application of a compound systemically and then measuring ABC transporter levels in the spinal cord would benefit the field.Up-regulation in response to chronic drug exposure has been shown at other blood-CNS barriers to occur in adults more readily than earlier stages of development (Koehn et al., 2019). Comprehensive studies of the regulatory capacity of the BSCB at early developmental stages are yet to be completed. An understanding of how concurrent medications may impact the likelihood of treatments accessing the spinal cord would be incredibly valuable. Such information would allow doctors to select patient's medications in a manner that would either not regulate transporters that might efflux CNS-directed medications or those that do up-regulate transporters to protect the spine against other systemically directed compounds.
The function of the BSCB can also be altered by injury or disease. The most obvious example of this is traumatic spinal cord injury where physical rupture of blood vessels forms a temporary breach of barrier integrity at the injury site where drugs up to 10 kDa in size can freely circumvent the normal barrier mechanisms and gain direct access to spinal tissue for up to 4 days post-injury (Koehn et al., 2016). It should be noted, however, that the site of damage might not always be the optimal active site for medications following such an injury. For example, a drug may need to access the cell body of motor neurons that are located at the rostral end of the spinal cord in the brainstem rather than at the site of axonal damage. Spinal cord injury is also known to increase ABC efflux transporter expression and function. A study by Dulin et al. (2013) in adult rats showed that at both 3 days and 10 months post-injury there was a persistent up-regulation in PGP expression, associated with a reduced spinal bioavailability of intraperitoneal injection of PGP substrate drug riluzole. RNAseq datasets in the adult mouse have also shown up-regulation ofabcb1b(PGP) 7-days post-injury by over 2 fold, along with a 44-fold increase inabcc3(MRP3; Chen et al., 2013). This information should be noted by drug developers, as the access of PGP and MRP3 substrate drugs to the spinal cord in uninjured animal models may not reflect the amount that can access the spinal cord following injury.
In summary, the addition of medications to treat the spinal cord must be considered in terms of the specific patient receiving the drug. Personal factors of the patient (e.g., age)could be very important in how likely particular ABC efflux transporters are to prevent drug transfer. In addition, the specific ailment of the patient as well as any other medications being taken by that patient could change the dynamic expression of ABC transporter defenses on barrier cells.Combined, these studies form a detailed understanding of the drug types likely to be restricted from transferring directly from the blood to the spinal cord tissue of patients.
The blood-cerebrospinal fluid and cerebrospinal fluid-spinal cord barriers
The direct transfer of a systemically derived drug from the spinal cord vasculature to the spinal tissue is not the only means by which a drug can access the spinal cord. The CSF from the fourth ventricle enters the subarachnoid space of the spinal meninges external to the spinal cord, with a small proportion entering the central canal running within the spinal cord (Vernau et al., 2008; Figure 1). The CSF plays a range of vital roles for the CNS including physical protection from trauma and molecular exchange of nutrients and waste products (Tashjian et al., 2019). Likewise, any drug that can move from the blood to the CSF has the potential to access spinal tissue from the CSF. This means that there are in fact two more important barriers regarding drug transfer to the spinal cord that need to be considered: the blood-CSF barriers and the CSF-spinal cord barriers (Figure 1).
The importance of the blood-CSF barriers in preventing molecular transfer is highlighted by examples of intrathecal drug administration having greater effects if allowed to bypass these barriers. As described in the introduction, intrathecally administered dyes can stain the spinal cord and intrathecal poisons can cause spinal toxicity in a manner that they could not via systemic application (Lewandowsky,1900; Goldman, 1909, 1913). In modern times a range of drugs including analgesics, are applied directly to the spinal cord to increase bioavailability of the drug at the active site(Bernards, 2002).
The most widely studied blood-CSF barrier cells are those that produce cerebrospinal fluid, the choroid plexuses. The barrier permeability of these epithelial cells have been described in detail, they are known to be functionally tight and prevent paracellular transfer from the earliest stages of its development (M?llg?rd and Saunders 1986; Johansson et al.,2008). ABC efflux transporters have been described in this tissue in both animal models (Gazzin et al., 2008; Ek et al.,2010; Liddelow et al., 2012; Kratzer et al., 2013) and humans(Daood et al., 2008; M?llg?rd et al., 2017) suggesting thatabcc1(MRP1) is a major transporter at this tissue. Studies have also suggestedabcc4(MRP4) is highly expressed at the choroid plexus, withabcb1(PGP) andabcg2(BCRP)described to varying extents between studies and species(Daood et al., 2008; Gazzin et al., 2008; Ek et al., 2010;Kratzer et al., 2013; M?llg?rd et al., 2017). The transporter profile of the principle blood-CSF barrier, the choroid plexus, has been described in detail in multiple reviews (Strazielle and Ghersi-Egea, 2015; Saunders et al., 2016).
In addition to the choroid plexus, there are blood-CSF and CSF-spinal cord barriers at the level of the meninges, preventing transfer between blood, the CSF of the subarachnoid space and the spinal tissue. Electron microscopy has shown that the arachnoid layer of the spinal meninges has tight junctions, which prevent molecular transport from the blood to the arachnoid CSF (Vandenabeele et al., 1996). Studies in the mouse have shown the presence of PGP at this barrier interface, adding examples of ABC efflux transporters at the blood-CSF barriers of the spinal arachnoid (Yasuda et al.,2013).Ex vivoanalysis of monkey and dog spinal meninges suggest that drugs (such as morphine) are prevented from entering the spinal cord by the arachnoid layer, which was nearly the sole rate-limiting layer for drug exchange across the meninges (Bernards and Hill, 1990).
Studies in the brain have shown that ABC efflux transporters exist at the ventricular barrier between CSF and brain in an age dependent manner (Koehn et al., 2019). The barriers between the CSF and the spinal cord, however, are not well understood. These include the ependymal cells lining the central canal of the spinal cord as well as the pial separation between the arachnoid CSF and the spinal cord. The cells of the adult rat central canal are separated by gap junctions,with studies in the cat describing an absence of PGP staining in these cells (Bruni and Reddy, 1987; Van der Heyden et al., 2009). These results correlate with other ependymal CSF-CNS interfaces such as the ventricular ependyma in the brain, although the MRPs present at the ventricular interface have not been fully examined at the central canal (Koehn et al., 2019). The study by Bernards and Hill (1990), described above, suggest that the pial membrane separation between the CSF and the spinal cord is highly permeable to morphine, unlike the arachnoid layer. This suggests that the same degree of molecular exclusion is not present at this layer as there is in the arachnoid cells, sharing similarities with what is known at the meningeal layer of the brain (Saunders et al.,2016).
There are a host of barrier interfaces that contribute to the transfer of a medication to the spinal cord via the CSF. These have been studied to varying extents, with ABC efflux transporter expression described at the choroid plexus in much greater detail than those in the central canal or spinal meninges. MRP (abcc) transporters may play a vital role in the prevention of molecular transfer to the CSF at the choroid plexus tissue, while PGP (abcb1) is one of the only transporters to be studied in detail at the spinal meninges (Yasuda et al., 2013). In order to fully understand molecular transport between the blood and spinal cord, the levels of efflux transporter expression must be well defined at the blood-CSF,CSF-spinal cord and BSCB.
Concluding Remarks
The present review describes the current literature regarding ABC efflux transporters at barriers between the blood and the CNS with a focus on how their presence may be beneficial or harmful in the treatment of spinal disorders. Principal ABC efflux transporters (PGP, BCRP, MRPs) are known to exist at these barriers and their expression is dependent on species, age, prior molecular exposure and injury/disease.While this information forms a beneficial background for future studies, the field of spinal cord barrier function is lacking behind the blood-brain barrier field in terms of specific ABC transporter expression and function and requires further investigation to fully inform patients, drug developers and medical practitioners.
The field would benefit from a study analyzing the presence or absence of all major ABC efflux transporters at the blood-spinal cord, blood-CSF and CSF-spinal cord barriers.Investigation of how each of these barriers alter expression levels over development, following chronic molecular exposure and between species would provide integral information to the field that has to this point largely focused on the BSCB. Finallyin vivomeasurements of the transfer of substrate medications for each ABC efflux transporter type would indicate the combined capacity of the system to restrict molecular transfer to the spinal cord, rather than relying onex vivoanalysis of one barrier type (e.g., spinal capillaries or spinal meninges).
As research in this space expands it is important that the field also considers the potential impact of other factors in molecular transport the spinal cord other than the overall levels of PGP, BCRP and MRPs. This may include studies of other ABC transporters (e.g.,abca), other transporter families (e.g., OATPs) and other transporter types that are yet to be conclusively characterized. Expansion of analysis to investigate which vessel subtypes these transporters are present on (capillary, artery, vein) would benefit future research as well as a detailed analysis of their cellular distribution to see if there are differences in transporter levels at different spinal segments.
The present review has highlighted the major impact that efflux transporters may play in both protecting the spinal cord from damage as well as preventing spinal recovery by reducing access of systemically applied medications. What is needed is to improve our understanding of the impact that alterations in efflux transporter functionality may result in. For example, the described increase in PGP (abcb1)expression following spinal injury (Chen et al., 2013; Dulin et al., 2013) may be playing a vital role in the protection of the highly vulnerable damaged tissue against naturally occurring molecules. Therefore, any attempts to decrease transporter expression during this time to allow more of a spinal treatment-specific drugs to access the tissue may have unexpected negative consequences, preventing the inherent protection provided by these transporters.
Researchers of molecular transfer into the CNS have begun to better understand the complexities of efflux transporter expression and function at the barriers between the peripheral circulation and the brain and spinal cord. It is now time for the field of spinal cord injuries/disorders to utilize this information to determine the safest medical practice for their patients both peripherally and at the spinal cord directly.
Author contributions:The author completed the manuscript independently and approved the final manuscript.
Conflicts of interest:The author declares no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Renata Ciccarelli, University of Chieti-Pescara,Italy; Jianxun Ding, Chinese Academy of Sciences, China;Mitsuhiro Enomoto, Tokyo Medical and Dental University, Japan.
Additional file:Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Glial cells in intracerebral transplantation for Parkinson's disease
- Fast-tracking regenerative medicine for traumatic brain injury
- The N-formyl peptide receptors: contemporary roles in neuronal function and dysfunction
- Adrenomedullin: an important participant in neurological diseases
- Shifting equilibriums in Alzheimer's disease: the complex roles of microglia in neuroinflammation,neuronal survival and neurogenesis
- Biomaterials and neural regeneration