Biomaterials and neural regeneration
Neural regeneration is a great clinical challenge in both the central nervous system (CNS) and the peripheral nervous system (PNS).Degeneration in the CNS caused by disease (such as Alzheimer's or Parkinson's diseases) or trauma (such as traumatic brain injury and spinal cord injury) often results in permanent paralysis and loss of sensation. The spontaneous neural regeneration in the CNS is generally unsuccessful, while the PNS has an intrinsic regenerative ability. However, PNS regeneration occurs over relatively short distances. Damage to the PNS results in the motor and cognitive impairment in many cases. Biomaterials in the form of scaffolds or nanoparticles for neuroprotection and neuroregeneration have attracted much attention. Nanoparticles can be fabricated using various lipids, polymers, metals, and carbon materials to carry drugs or growth factors. Here, biomaterial scaffolds for neural regeneration are highlighted. Some certain criteria for biomaterial scaffolds in neural regeneration should be met, for example, the biocompatibility for integration with the host tissues, the degradation rates close to the nerve growth, and the mechanical properties similar to the nerve tissue (Hsieh et al., 2015). A number of natural and synthetic polymers have been applied in neural regeneration, including collagen, gelatin, chitosan, alginate, hyaluronan, silk fibroin, poly(L-lactic acid), poly(glycolic acid), polycaprolactone, polyphosphoester,and polyurethane. To improve the bioactivity, conductive biomaterials such as polypyrrole, polythiophene, and polyaniline may enhance neurite outgrowth because electrical signals are transmitted for neuronal communication (Guo and Ma, 2018). Mechanical properties of biomaterial scaffolds can influence neural regeneration, where the soft materials (0.1-1 kPa) promote the neuronal differentiation but the stiffer materials (7-10 kPa) promote the glial differentiation (Tseng et al., 2015). Hydrogel and nerve guide conduit (NGC) are the most frequent choices of scaffolds in CNS and PNS regeneration (Figure 1A). Moreover, self-healing injectable hydrogel and three-dimensional (3D) printed NGC with unique properties are attractive candidates in each category.
Hydrogels in neural regeneration:Hydrogels, highly water-absorbent crosslinked polymer networks, have been broadly developed as the injectable scaffolds for CNS regeneration. Drug delivery and cell transplantation are the current main strategies to treat CNS injury and disease. Injectable hydrogels can deliver drugs/cells to the target sites. Besides, the glial scar formation acts as the major barrier to the regeneration of axons after injury or disease to the CNS(Cregg et al., 2014). To overcome this problem, injectable hydrogels can fill in the irregular voids for the structural support, and provide a permissive microenvironment for the axonal growth. However,the conventional injectable hydrogels are limited by the difficulty of controlling the gelation process. These hydrogels can pass through the needle and undergo gelation at the target site. Fast gelation may result in clogging of the needle, while slow gelation may lead to loss of hydrogel from the target site. Meanwhile, self-healing hydrogels based on reversible crosslinks can be conveniently injected after gelation, and therefore have better mass retention. Self-healing hydrogels should own the ability to reform shapes and recover functions under physiological conditions. Among various self-healing mechanisms, the Schiff base linkage, ureidopyrimidinone quadruple hydrogen bond, catechol-iron interaction, and host-guest interaction are employed to prepare injectable self-healing hydrogels for evaluation by animal models (Liu and Hsu, 2018). Literature reveals these self-healing injectable hydrogels may be promising materials for CNS regeneration in the future. A recent study revealed that oxygen metabolism and neural differentiation of neural stem cells (NSCs) in the hydrogel were positively correlated with the self-healing ability of the hydrogel (Cheng et al., 2019).
NGCs in neural regeneration:NGC, a tubular scaffold to connect two severed nerve stumps, was developed to replace the autologous nerve grafts for PNS regeneration. NGCs are applied to enhance the repair of long-distance peripheral nerve defects where the axons need to extend across the long nerve gap and connect to the distal nerve segment. The major concerns of an ideal NGCs include biocompatibility, biodegradability, biomechanical properties, permeability, and surface properties. The sufficient permeability of NGCs could promote the nutrient and gas exchange, allow capillary ingrowth, and prevent fibrosis of the repair site by maintaining a barrier to fibroblasts and other cells (Gu et al., 2011). Chemistry and topography of material surface can influence cell attachment, proliferation, and differentiation. The appropriate surface properties favor the uniform alignment of cells and the directional growth of axons to improve the PNS regeneration. Moreover, complex structures are introduced into the NGC lumens, such as filaments-filled NGCs, sponge-filled NGCs, and multichannel NGCs, for improvement of functional outcomes in nerve repair. 3D printing (additive manufacturing) is an effective method to fabricate NGCs with desired surface permeability and architectural structures, and at the same time incorporate cells and growth factors into the NGCs. The 3D printed NGCs can be prepared based on microscale structural units to mimic the peripheral nerve architecture with various biomaterials, biomolecules, and cells. In addition, 3D printed NGCs can be created as patient-specific nerve conduits through advanced bioimaging techniques. 3D printed NGCs show the great potentials of the PNS regeneration toward precision medicine.
Cells and growth factors in neural regeneration:Cells and/or growth factors are often incorporated into the biomaterial scaffolds for improvement of nerve regeneration. Cells implanted into the injured nerve can produce growth factors and extracellular matrix.Neural and other stem cells are widely investigated in both CNS and PNS regeneration, while Schwann cells are generally used in PNS regeneration. Formation of the cellular spheroids could facilitate cell-cell interaction and cell-extracellular matrix interaction, which leads to enhancement of physiological properties and therapeutic potentials of cells (Han and Hsu, 2017). Single-cell and multi-cell spheroids can be conveniently prepared by culturing the cells on the chitosan or hyaluronan-grafted chitosan substrates.The cells in the spheroids show higher proliferation rate and differentiation potential compared to the dissociated cells. In a recent study, NSCs and endothelial cells (ECs) were assembled into the NSC/EC co-spheroids by co-culturing on hyaluronan-grafted chitosan substrates (Han et al., 2019). NSCs in the NSC/EC co-spheroids exhibited greater differentiation potential compared to that in the NSC spheroids, and then the NSC/EC co-spheroids formed a neurovascular unit-like structure in the gelatin-based hydrogel. The synergistic effect of the NSC/EC co-spheroids on the neural differentiation and the angiogenic activity reveals that the formation of multi-cell spheroids is a potential strategy for cell therapy in neural regeneration. Moreover, exogenous growth factors can be added into the biomaterial scaffolds to regulate cell proliferation, migration, and differentiation. The continuous and controlled delivery of growth factors from the biomaterials is investigated extensively to provide the basic components directly during the period of the CNS regeneration or the PNS regeneration. The commonly used growth factors include neurotrophic factors (i.e., nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5) and other growth factors (e.g., glial cell line-derived neurotrophic factor, ciliary neurotrophic factor, and fibroblast growth factors).
Vascularization in neural regeneration:Vascularization plays a fundamental role in the neural regeneration of both CNS and PNS.The neural tissues are vascularized early in the regeneration for the adequate supply of blood, oxygen and other nutrients. The vascu-lar network tissue can form by co-culturing cells, incorporating growth factors, or fabricating channels inside the hydrogel using sacrificial materials. Tubular channels in hydrogel construct can form conveniently using a glucose-sensitive sacrificial material that is effectively removed by immersion in the culture medium (Tseng et al., 2017). In this case, ECs may form the capillary-like structure in the channels while NSCs form neurosphere-like structure in the construct, showing the morphology of a vascularized neural tissue.In the future, the cellular co-spheroids may be printed with materials to fabricate the construct with channels using 3D bioprinting.
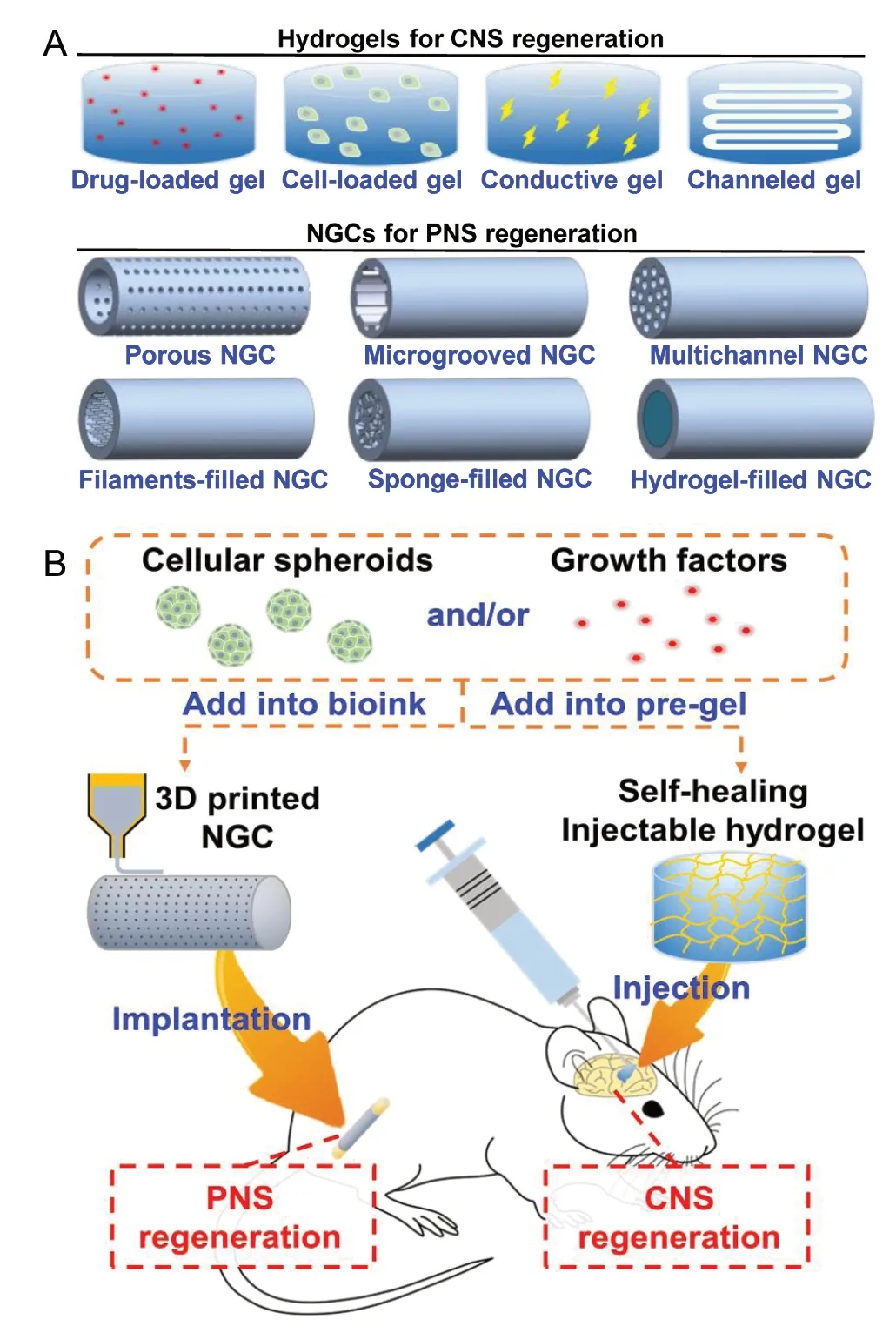
Figure 1 Biomaterials in neural regeneration.(A) Examples for hydrogels and NGCs used in CNS and PNS regeneration. Hydrogels are developed in CNS regeneration, such as drug-loaded hydrogels, cell-loaded hydrogels, conductive hydrogels, and channeled hydrogels. NGCs are applied in PNS regeneration, including porous NGCs, microgrooved NGCs, sponge-filled NGCs, multichannel NGCs, filaments-filled NGCs, and hydrogel-filled NGCs. (B) The potential strategies of biomaterial scaffolds for regeneration of the CNS and the PNS. Cells or preferably cellular spheroids are prepared using neural- and vascular-related cells. The cells (or cellular spheroids) and growth factors are added into the pre-gel solutions for the preparation of self-healing injectable hydrogel or the fabrication of 3D printed NGC. Self-healing hydrogel can be injected into the injured site of the brain or spinal cord through a long narrow needle for the CNS regeneration. 3D printed NGC can be implanted to bridge a peripheral nerve gap by surgical operation for the PNS regeneration. 3D: Three-dimensional; CNS: central nervous system; NGC: nerve guide conduit; PNS:peripheral nervous system.
Summary:Biomaterial scaffolds used in neural regeneration have been developed as important therapeutic strategies, especially for those incorporated with cells and/or growth factors (Figure 1B).Self-healing injectable hydrogels and the 3D printed NGCs are in the frontier for the CNS regeneration and the PNS regeneration.Cells, including neural- and vascular-related cells, are desired to form spheroids or co-spheroids (or even organoid) before being embedded in the biomaterials. Growth factors provide extra temporal-spatial features, e.g., sustained release in the appropriate site.A few other concerns should be considered for design of the therapeutic strategies, including structural and functional similarity of regenerating tissue to the native nerve tissue, inhibition of scar formation, and enhancement of vascularization.
This work was supported by the Ministry of Science and Technology(MOST 108-2321-B-002-047) and the National Taiwan University(NTU-CC-108L891101).
Yi Liu, Shan-hui Hsu*
Institute of Polymer Science and Engineering, National Taiwan University, Taiwan, China (Liu Y, Hsu SH)
Institute of Cellular and System Medicine, National Health Research Institutes, Miaoli, Taiwan, China (Hsu SH)
*Correspondence to:Shan-hui Hsu, PhD, shhsu@ntu.edu.tw.
orcid:0000-0003-3399-055X (Shan-hui Hsu)
Received:June 10, 2019
Peer review started:September 30, 2019
Accepted:November 13, 2019
Published online:January 6, 2020
doi:10.4103/1673-5374.272573
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Glial cells in intracerebral transplantation for Parkinson's disease
- Fast-tracking regenerative medicine for traumatic brain injury
- The N-formyl peptide receptors: contemporary roles in neuronal function and dysfunction
- Adrenomedullin: an important participant in neurological diseases
- Shifting equilibriums in Alzheimer's disease: the complex roles of microglia in neuroinflammation,neuronal survival and neurogenesis
- ABC efflux transporters at blood-central nervous system barriers and their implications for treating spinal cord disorders